howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Acute Lymphoblastic Leukemia/ Lymphoma
Introduction:
- It is a group of malignancies arising for lymphoblasts which are committed to B or T cell lineage.
Epidemiology:
- It comprises of
- 12% of all cases of leukemias
- 80% of all childhood leukemias
- 20% of adult leukemias
- T-ALL comprises 15% of childhood ALL and 25% of adult ALL
- Incidence: 1.6/1lac population
- Median age- 13 years (Peak- 2-4 years)
- 60% cases <20 years, 24% cases are >45 years
Etiology: Exact etiology is not known in most of the cases
- Congenital syndromes
- Down syndrome- Usually associated with hyperdiploidy (>50) and t (12:21)
- Ataxia telangiectasia
- Bloom syndrome
- Nijmegen breakage syndrome
- Prenatal Origin of ALL: Evidences for this include
- MLL – AF4 & TEL – AML1 fusion genes are identified in neonatal blood spots
- Development of concordant leukaemia in twins
- Environmental factors
- Use of topoisomerase 2 inhibitor is associated with mutations of MLL genes on chromosome 11q23
- Radiations- Especially in utero exposure
- Transplacental foetal exposure to following drugs is associated with MLL rearrangements- Flavonoids, Quinolones, Benzene metabolites, Catechins, Podophyllin resins, Oestrogens, Herb medicine, Pesticides
- Genetic deficiency of carcinogen detoxifying enzymes. Ex: Glutathione S transferase, Nicotinamide adenine nucleotide phosphate quinoneoxidoreductase
- Immunodeficiency states
- Congenital immunodeficiency syndromes- Wiskott Aldrich syndrome, congenital hypogammaglobulinemia, ataxia telangiectasia
- Chronic immunosuppressive therapy: This leads to loss of tumour immune surveillance and proliferation of malignant clone of cells.
- Delayed infection hypothesis:
- Susceptible individuals with prenatally acquired pre-leukemic clone, if not exposed to infections early in life (usually because of affluent hygienic environment), lymphoid cells remain in precursor lymphoid cell stage.
- Infection at a later age (2-4 years), when there is increased lymphoid proliferation, leads to aberrant response, which results in uncontrolled proliferation of lymphoid cells.
Pathogenesis:
Translocations, Inversions, Deletions, Point mutations, Amplifications
↓
Multistep somatic mutations in single lymphoid progenitor cell
1. Aberrant expression of proto-oncogenes- IK2F1, MYC, TAL1, LYL1, LMO2, HOX11
2. Genes encoding active kinases: BCR – ABL, CSF 1R, JAK2, PDGFRB, RAS
3. Transcription factors (TEL – AML1, E2A – PBX1, MLL linked to many fusion partners)
4. Tumor suppressor and cell cycle regulation genes: TP53, RB1, CDKN2A/CDKN2B, CDK Inhibitors, FBW 17, LEF1, BCL 11B, PHF 6
5. Cytokine receptor genes: CRLF2, EPOR, IL7R
6. Epigenetic modifications: EZH2, CREBBP, SETD2, MLL2, NSD2
↓
1. Subversion of the controls of normal proliferation
2. Block in differentiation
3. Resistance to death signals (Apoptosis)
4.Enhanced self-renewal
↓
Uncontrolled, limitless proliferation of precursor lymphoid cells
WHO Classification:
- B-lymphoblastic leukaemia/lymphoma
- B-lymphoblastic leukaemia/lymphoma with high hyperdiploidy
- B-lymphoblastic leukaemia/lymphoma with hypodiploidy
- B-lymphoblastic leukaemia/lymphoma with iAMP21
- B-lymphoblastic leukaemia/lymphoma with BCR::ABL1 fusion
- B-lymphoblastic leukaemia/lymphoma with BCR::ABL1-like features
- B lymphoblastic leukaemia/lymphoma with KMT2A rearrangement
- B lymphoblastic leukaemia/lymphoma with ETV6::RUNX1 fusion
- B-lymphoblastic leukaemia/lymphoma with ETV6::RUNX1-like features
- B lymphoblastic leukaemia/lymphoma with TCF3::PBX1 fusion
- B lymphoblastic leukaemia/lymphoma with IGH::IL3 fusion
- B lymphoblastic leukaemia/lymphoma with TCF3::HLF fusion
- B-lymphoblastic leukaemia/lymphoma with other defined genetic alterations
- B-lymphoblastic leukaemia/lymphoma, NOS
- T-lymphoblastic leukaemia/lymphoma
- T-lymphoblastic leukaemia/lymphoma- NOS
- Early T-cell precursor lymphoblastic leukaemia/lymphoma
Clinical Features:
- Due to marrow depression
- Anaemia- Fatigue, dizziness, palpitations
- Neutropenia – Frequent infection with fever
- Thrombocytopenia – purpura and haemorrhages
- Due to marrow expansion and leukemic cell infiltration into periosteum and joints
- Bone pain
- Joint pain
- Pain in extremities
- Due to organ infiltration
- Mediastinal mass due to thymic involvement
- Lymphadenopathy
- Splenomegaly
- Hepatomegaly
- Due to meningeal spread (CNS involvement)
- Headache
- Vomiting
- Nerve palsies – especially facial and abducent
- Papilledema
- Due to testicular involvement
- Swelling of testis
- Hydrocele can be there due to lymphatic obstruction
- Due to release of cytokines such as IL1, IL6 and TNF alpha
- Fever- It resolves within 72hrs of starting antileukemia therapy
- Rarely patients can have following features
- Subcutaneous nodules (leukaemia cutis)
- Enlarged salivary gland
- Priapism- Due to leukostasis of corpora cavernosa and dorsal vein. It can be due to involvement of sacral vein as well.
- Ocular involvement: Leukemic infiltration of orbit, optic nerve, retina, iris, cornea or conjunctiva
- DIC
- Renal involvement: due to tumour lysis syndrome/ renal infiltration
- Hypercalcemia due to release of PTH like protein
- Liver dysfunction: Due to leukemic infiltration
- Leucostasis: Large number of lymphoblasts in peripheral circulation leading to respiratory distress, altered mental status
Investigations:
- Hemogram
- Normocytic normochromic anemia
- Increased number of lymphoblasts
- Neutropenia
- Thrombocytopenia
- Morphology of lymphoblasts (FAB classification)
Morphologic Features | L1 (Usually seen in children) | L2 (Usually seen in adults) | L3 (Burkitt type) |
Cell size | Small | Large | Large |
Nuclear chromatin | Fine or clumped | Fine | Fine |
Nuclear shape | Regular, may have cleft or indentation | Irregular, may have cleft or indentation | Regular, oval to round |
Nucleoli | Indistinct or not visible | One or more per cell large, prominent | One or more per cell; large, Prominent |
Amount of cytoplasm | Scanty | Moderately abundant | Moderately abundant |
Cytoplasmic basophilia | Slight | Slight | Prominent |
Cytoplasmic vacuoles | Variable | Variable | Prominent |
- Bone marrow aspiration
- Lymphoblast count ranges from 60% to 100%
- Bone marrow biopsy
- Packed with lymphoblasts
- Trilineage hematopoiesis is markedly suppressed
- Lymph node biopsy
- Diffuse pattern of involvement by lymphoblasts
- Cytochemistry
- MPO; SBB – Negative
- PAS – Positive (Chunky/ Block positivity)
- NSE – Multifocal punctuate pattern
- Immunophenotyping (Flow cytometry/ Immunohistochemistry)
- B-ALL
- Usually positive for: CD19, CD22, CD79a, CD10, CD34, CD45, HLADR, TDT
- Some are positive for: CD20, CD13, CD33, CD56,
- By definition negative for: CD3, MPO
- T-ALL
- Usually positive for: CD3, CD5, CD2, CD7, CD1a,CD34, CD45, TDT
- Some are positive for: CD10, CD13, CD33, HLADR
- CD4 and CD8- Monoclonal expression (frequently co-expressed)
- By definition negative for: MPO, CD19
- B-ALL
- Cytogenetics
- Preferably to be done from bone marrow sample
- Important in assessing the prognosis
- Even if cytogenetics is done, molecular tests (FISH/PCR) for specific translocations has to be sent, as some of these are cryptic and some may be missed due to technical errors.
- Ploidy classes-
- High hyperdiploidy>50, but <66 chromosomes- Good prognosis
- Hyperdiploidy 47 – 50 chromosomes
- Normal karyotype
- Pseudodiploidy (Normal number of chromosomes with structural changes)
- Hypodiploidy < 46 chromosomes- Associated with poor prognosis
- Near triploidy : 69 – 81 chromosomes
- Near tetraploidy : 82 – 94 chromosomes
- Complex karyotypes: 5 or more chromosomal abnormalities- Poor prognosis
- CSF analysis
- Done at diagnosis to note CNS involvement (postpone lumbar puncture if there is severe thrombocytopenia)
- Intrathecal chemotherapy should be administered after collecting CSF sample
- Sample should be sent for cytocentrifuge smears
- Involvement is seen in about 1/3rd of patients at diagnosis or during the course of treatment
- Terminology for CNS involvement
- CNS 1 (Negative)- No lymphoblasts in CSF regardless of WBC count. No clinical/radiological evidence of CNS disease.
- CNS 2 (Negative)- WBC <5/cmm with presence of lymphoblasts
- CNS 3 (Positive)- WBC ≥ 5/cmm with presence of lymphoblasts
- In case of hemorrhagic tap with presence of blasts in peripheral blood and >5 WBC/cmm cells in CSF, follow Steinherz- Bleyer algorithm (Compare CSF- WBC/RBC ratio to Blood- WBC/RBC ratio)
- If CSF ratio is at least 2 fold greater than blood ratio- Classify it as CNS 3
- If not- Classify it is CNS 2
- Cranial nerve palsy, fundoscopy showing tumor deposits, intracranial mass and leptomeningeal involvement in MRI are also considered as CNS 3.
- LFT, RFT, Ca, PO4, Uric acid, LDH- As routine pretreatment work up
- Chest X ray/ CT thorax- To know mediastinal involvement (in case of T ALL)
- 2 D Echo- As anthracyclines are used in the treatment
Criteria for Diagnosis:
- Any percentage of lymphoblasts with typical immunophenotypic features on flow cytometry or immunohistochemistry. But usually, number of blasts is >20%.
Prognosis:
- Over all cure rate:
- Children: 80%
- Adults: 30-40%
- Favourable risk factors
- White blood cell count- <30 x 109/L
- Age- 3 – 7 yr
- Gender- Female
- Ethnicity- White
- No Node, liver, spleen,testicular, CNS involvement
- FAB morphologic features- L1
- Immunophenophenotyping: Early Pre-B ALL, Dim CD 45 intensity
- Ploidy- Hyperdiploidy- between 51 & 65 chromosomes
- Cytogenetic markers- t(12;21) Fusion of TEL with AML1
- Gene expression profile- Mature type
- Time to remission- <14 d
- Minimal residual disease- Negative at the end of induction. Most groups consider <0.01% to be MRD negative. But there is no uniformity in assessment for MRD. Problems in MRD assessment include
- Different study groups use different cut off values
- Techniques and analysis softwares used for MRD are different
- Timing of MRD assessment are different in different protocols
- Induction chemotherapy includes different chemotherapy agents in different protocols
- MRD for one target population may not be applicable to another population of ALL
- Unfavourable risk factors
- White blood cell count- >100 x 109/L
- Age - <1 year, >10 years
- Gender- Male
- Ethnicity- Black
- Node, liver, spleen,testicular, CNS involvement
- Prednisolone poor response
- Day 14 and day 35 BM- M2 or M3 status
- FAB morphologic features- L2
- Immunophenophenotyping: Mature B ALL, T ALL, CD45- Bright, CD20- bright, ETP ALL
- Ploidy- Hypodiploidy<45
- Cytogenetic markers
- t(9;22)
- t(4;11) with fusion of MLL with AF4
- t(1;19) with fusion of E2A & PBX
- Mutations for genes: IKZF1, NUP214-ABL1, CRLF2, CREBBP, CRLF 2, TP53
- Gene expression profile- AML type, precursor cell type
- Time to remission- >28 days
- Minimal residual disease- Positive at end of induction
- Mediastinal disease which persists 1 week after end of induction and resected biopsy shows viable blasts
- Testicular disease that persists at day 33 and tumour cells found in biopsy
Differential Diagnosis:
- AML with minimal differentiation
- Hematogones of reactive bone marrow
- Seen in increased number in BM of children & old age individuals, iron deficiency anemia, neuroblastoma, ITP, following cytotoxic therapy and following viral infections
- Morphologically they look exactly like blasts with very high nucleocytoplasmic ratio. Nucleus has homogeneous chromatin, may show clefts and nucleoli are not conspicuous
- Never found in peripheral blood
- Difficult to differentiate them from leukemic B-lymphocytes as both are TdT& CD10 positive
- But they lack aberrant antigen expression & show a reproducible pattern of co-expression of markers associated with B lineage- CD10, CD19, CD20, CD34 & CD45
- There is smeared expression of CD20 and CD34. (Expression of CD20 and CD34/TDT are mutually exclusive)
- Cells with dim CD45 positivity are CD34 positive and cells with moderate CD45 positivity are CD34 negative.
- They have low side scatter compared to lymphoblasts
- B cell lymphoma with follicular center cell origin
- Bright CD45 and moderate to bright CD20 and surface Ig
- CD10 is dim
Pretreatment Work-up:
- History
- Examination
- LN:
- Spleen:
- Testis:
- Fundoscopy:
- WHO P. S.
- BSA
- IHC/Flow cytometry
- BMA and Bx
- CNS status (CSF for cytospin)
- D1-
- D33-
- Haemoglobin
- TLC, DLC
- Peripheral smear
- LFT: Bili- T/D SGPT: SGOT:
- Creatinine
- Electrolytes: Na: K: Ca: PO4:
- Uric acid
- LDH
- HIV:
- HBsAg:
- HCV:
- UPT
- Cytogenetics
- CT/ MRI of head (If CSF Positive/ CNS Symptoms)
- USG-
- Neck:
- Abdomen, Pelvis:
- Testis (if there is painless testicular enlargement)
- CXR PA/Lat or CT Chest in T-ALL (Mediastinal width at D5)
- D1-
- D33-
- FISH/ PCR
- BCR-ABL1
- MLL-AF9,
- MLL-AF4,
- MLL-ENL,
- t(12;21),
- t(1;19)
- ECHO- LVEF- %
- HLA Typing for HR patient
- TPMT Mutation
- If homozygous status 10% dose of 6MP is adequate.
- Heterozygous status need moderate reduction in dose.
- Protein C and S
- As there is increased risk of thrombosis with L-Asparginase
- Day 8 Steroid response
- Day 14 BMA
- Day 35 BMA
- Overall risk
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
Treatment Plan:
- For children <1year- Use Interfant protocol
- For old and frail patients- Give prednisolone and weekly vincristine to achieve CR. Then give 6MP + Methotrexate maintenance (along with Imatinib/ Dasatinib if Ph positive) till progression of disease.
- For other patients (paediatric, adolescent and fit adults)- Follow modified BFM 2002 protocol. (Use anthracyclines with caution in patients aged >40 years)
- For Ph-Positive patients, add Dasatinib/ Imatinib from phase 2 of induction. At CR1 explain option of Allo-SCT. If not willing/ Allo SCT is not possible, continue BFM protocol along with Imatinib/ Dasatinib. In view of high risk of CNS relapse, follow T-ALL protocol. After completion of maintenance, continue TKI lifelong)
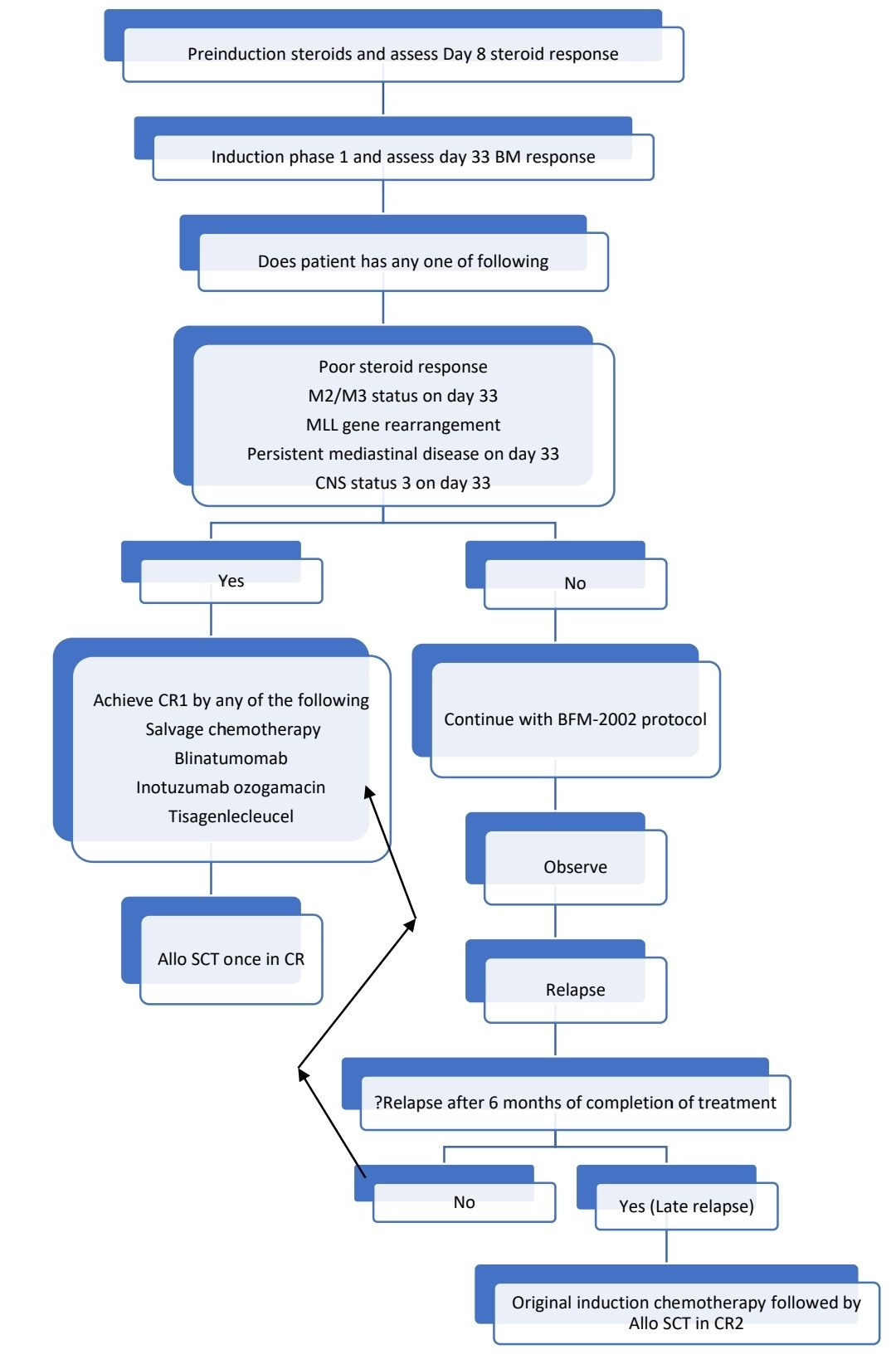
Overall outcome is much better when paediatric protocols are used in adults. Although use of L-asparaginase is associated with higher risk of pancreatitis, hepatic toxicity and venous thromboemboli in adults, its use is has shown potential survival benefit. Hence, L-asparaginase in slightly lower doses is better option, than altogether omitting a vital drug from the protocol.
For T- Acute lymphoblastic leukaemia, following changes are made:
- Triple IT may be given instead of IT Methotrexate
- Methotrexate dose during consolidation is 5,000mg/m2
- Cranial irradiation is given (prophylactic- 12Gy and therapeutic –18Gy) after completion of reinduction, prior to starting maintenance.
Other option in adults is HyperCVAD/MA Protocol
- 4 Cycles of Hyper-CVAD (Cycles-1,3,5,7)/MA with aggressive hydration (Cycles- 2,4,6,8) (i.e. 4cycles of A+B) then maintenance with 6-MP and MTX, monthly vincristine (1.4mg/m2) and Dexamethasone (6mg/m2- on 1st 5 days of every month) and 3 monthly IT-MTX for 2 years.
- If documented CNS disease- IT therapy twice weekly till CSF clears and then weekly for 4 weeks. Then continue IT as per protocol. In addition Cranial RT (24-30Gy) after completing 4 (A+B) cycles, prior to starting maintenance.
- For T ALL with mediastinal involvement- Mediastinal irradiation after intensive therapy, before maintenance. (30Gy over 3-4 weeks)
- Remission assessment and Follow up CT scan to be done after 2(A+B) cycles.
Modified BFM-2002 Protocol
Induction: Phase-1
- Tab. Prednisolone 60mg/m2 (Max-100mg) in 3 divided doses. Start at 25% dose on day 1 and increase to full dose by 3rd or 4th day. Taper from day 28 over next 7 days. Stop by day 35.
- Inj. Daunorubicin 30mg/m2 in 100 ml NS over 1hr- once a week for 4 weeks from day 8
- Inj. Vincristine 1.5mg/m2 (Max 2mg) in 100ml NS 15 min- once a week for 4 weeks from day 8
- Inj. L-asparginase- 5000 U/m2 in 100 ml N saline over 1 hour (12-hour post inj.Vincristine)- 8 doses- 2-3 days apart starting from day 12. OR Peg L Asp- 1000units/m2 in 100ml NS over 1hr- only 2 doses 2 weeks apart
- Inj. Methotrexate- IT- On days 1 and 12. (Total 4 doses if CNS Positive/Traumatic tap/TLC>50,000/cmm)
- Dose adjustments
- Ejection fraction- <35% - Avoid Daunorubicin
- Unequivocal pancreatitis (Abdominal pain with increased amylase or lipase and imaging evidence)- Omit- L-asparaginase
Induction: Phase 2
- Start only if:
- Good general status
- No severe infection
- Creatinine normal for age
- Recovering blood counts- TLC>2000, ANC>500, Platelets>50,000
- For second dose cyclophosphamide – TLC>1000, ANC> 300, Platelets>50,000, creat- normal
- If cytopenia or infection withhold cytosine block and 6-MP.
- Avoid interrupting a cytosine block
- Inj. Cyclophosphamide 1,000 mg/m2 in 500ml D5% over 2 h (IV hydration ½ NS 3,000 ml/m2/24h, MESNA 400 mg/m2 , IV, at: 0, +4 and +8 h) - 2 doses- 1st one at the starting of Phase 2 (day 36) and 2nd dose at the end (after 4th week of cytosine block)
- Inj. Cytarabine 75 mg/m2 /d, SC- OD for 4 days a week. For 4 weeks
- Then G-CSF- 5mcg/kg for remaining 3 days of the week
- Inj. Methotrexate- IT- Given at the beginning of each week (along with 1st dose of cytarabine)
- Tab. 6-Mercaptopurine 60 mg/m2 /d- day: 36 – 63 (28 days)- Generally not well tolerated, hence avoided.
15 days gap
Consolidation:
- Start only if
- Satisfactory general condition and no serious infections
- Creatinine is normal and no urinary obstruction
- AST/ALT- <5x ULN, Bilirubin- <3xULN
- Blood counts- TLC > 1500/cmm, ANC- >500/cmm, PL- >50,000/cmm
- No evidence of ascites or pleural effusion
- Inj. Methotrexate 2,000/ 5000 mg/m2 (Depending on phenotype and other CNS risk factors), IV over 24 h with IT MTX and leucovorine rescue- Every 2 weeks for 4 doses.
- Tab. 6-Mercaptopurine- 25 mg/m2/d, for 8 weeks- Generally not well tolerated, hence avoided.
- Stop Septran
- Strict I/O Monitoring. If Positive by >400ml/m2 in 12hrs-Give Inj. Frusemide-0.5mg/kg (max 20mg)- Stat
- Hydration for 6 hrs with NS or ½ NS with 4 amp Sodium Bicarbonate (125ml/m2/hr), which should be continued till last folinic acid injection.
- Urine pH- after 6 hrs of starting hydration- 6hrly- If pH is <7, give Inj. NaHCO3 stat (2-6 ampoules) and recheck pH after 3 hrs
- Start chemotherapy once urine pH>7.
- Loading dose: Inj. Methrotrexate (10% of total dose) in 250ml NS over 30 min.
- Infusion dose: Inj. Methotrexate (90% of total dose over 23hrs)
- Inj. Methotrexate- Intrathecal- Preferably given when methotrexate infusion is going on.
- Folinic acid (15mg/m2) rescue started 42 hours after starting Methotrexate. Given 6 hrly for total of 6 doses.
- For 5gm/m2, monitoring methotrexate levels is desirable but not mandatory.
Time of methotrexate infusion | Cut off methotrexate level (micromol/L) |
24hrs | 150 |
36hrs | 3 |
42hrs | 1 |
48hrs | 0.4 |
54hrs | 0.4 |
- Continue Leucovorine rescue till MTX levels fall to <0.25 micromol/L
- Routine assessment of serum methotrexate levels is not necessary if 2gm/m2 is given. (Do it only if there is oliguria/ hypertension/ edema/ weight gain/ emesis/ confusion/ blurred vision/ increased creatinine- If high forced dieresis, alkalization, stringent fluid balance monitoring, Adequate leucovorin rescue (Dose= Plasma MTX level x Body weight, If dose is >20mg/kg, give over 1hr, to avoid calcium induced bradycardia), monitor daily Creatinine, sodium and potassium. Alternate days Bilirubin, ALT, albumin, full blood count.
- W/F Acute encephalopathy- Details available in supportive care section.
15 days gap
Reinduction: Phase 1
- Start only if
- Satisfactory general condition and no severe infections
- Start if TLC>2500, ANC>1000, Platelet count >1,00,000
- If TLC<1000 / ANC<500 – postpone doxorubicin and vincristine
- In case of severe neuropathy stop vincristine
- Avoid Adriamycin if EF is <35%
- If pancreatitis seen – omit L- asparaginase
- Tab. Dexamethasone 10mg/m2 divided doses- From day 1 to day 21, then taper over 10 days
- Inj. Doxorubicin 30mg/m2 in 100 ml NS over 1hr- once a week for 4 weeks from day 8
- Inj. Vincristine 1.5mg/m2 (Max 2mg) in 100ml NS 15 min- once a week for 4 weeks from day 8
- Inj. L-asparaginase- 10,000 U/m2 in 100 ml N saline over 1 hour (12-hour post Inj. Vincristine)- 4 doses OR Peg L Asp- 1000units/m2 in 100ml NS over 1hr- only 2 doses 2 weeks apart
- Inj. Methotrexate- IT- On days 8 and 22.
Reinduction: Phase 2
- Start only if:
- Satisfactory general condition and no severe infections
- Normal creatinine
- Blood count TLC>2000, ANC>500, Platelet count>50,000
- Avoid interrupting run block of cytosine, if needs to be stopped, stop 6- TG
- Inj. Cyclophosphamide 1,000 mg/m2 in 500ml D5% over 2 h (IV hydration ½ NS 3,000 ml/m2/24h, MESNA 400 mg/m2, IV, at: 0, +4 and +8 h) - 1 dose- on day 36
- Inj. Cytarabine 75 mg/m2 /d, SC- OD for 4 days a week. For 2 weeks
- Then G-CSF- 5mcg/kg for remaining 3 days of the week
- Inj. Methotrexate- IT- Given at the beginning of each week (along with 1st dose of cytarabine)
- Tab. 6-Thioguanine 60 mg/m2 /d- day: 36 – 49 (14 days)- Generally not well tolerated, hence avoided.
15 days gap
Maintenance:
- To be given for 2 years 6 months
- Stop if:
- Poor general condition or severe infection.
- WBC count<1000/cmm
- SGPT/SGOT > 5xULN or Bilirubin: >3x ULN
- Long standing diarrhea / pneumonia
- Altered creatinine
- Start with 50% dose and then increase to up to 150% of dose, to maintain TLC between 2000-3000/cmm.
- Tab. 6 Mercaptopurine- 350mg/m2/week- divided over 1 week (To be taken in evening on empty stomach with no milk/ milk products)
- Tab. Methotrexate- 20mg/m2- Once a week
- Give Septran and Acyclovir prophylaxis.
- Inj. Methotrexate- IT- Once in 3 months
WBC count (/cmm) | % of 6-MP &Methotrexate dose |
<1000 | 0% |
1000-2000 | 50% |
2000-3000 | 100% |
>3000 | 150% |
Some centres give following additional treatment during maintenance
- Tab. Dexamethasone- 6mg/m2 - OD- for 5 DAYS in a Month
- Inj. Vincristine- 1.4mg/m2 (Max-2mg) - Once a month
Follow up after completion of maintenance: Once in 3 months for 2 years.
Consolidation as per G-MALL protocol
2 cycles- each cycle- 28 days
Inj. Cytarabine- 75mg/m2- in 100ml NS over 1hr- From day 1 to day 5.
Inj. Etoposide- 50mg/m2- in 500ml NS over 3hrs- From day 1 to day 5.
Intrathecal chemotherapy- On day 1.
HYPER-CVAD Protocol
PART- A
Frequency: 21 days
Protocol:
- Inj. Cyclophosphamide- 300mg/m2 in 500ml D5% over 2 hrs- BD- From Day 1 to Day 3 (Total 6 doses)
- Inj. Mesna- 300mg/m2 in 500ml D5% over 12 hrs- BD- Start 1 hr prior to cyclophosphamide- From Day 1 to Day 3 (Total 6 doses)
- Inj. Doxorubicin- 50mg/m2 in 500ml NS over 48hrs- On day 4 (Single dose)
- Inj. Vincristine- 1.4mg (Max- 2mg) in 100ml NS over 10min- On Day 4 and Day 11.
- Inj. Dexamethasone- 40mg in 100ml NS over 30min- From Day 1 to Day 4 and from Day 11 to Day 14.
- Intrathecalmethotrexate- 12mg—on day 2
- Intrathecal cytosine- 100mg- On day 8
- G-CSF- 300mcg- SC-OD from Day 5
Dose adjustments:
|
| Cyclophosphamide | Doxorubicin | Vincristine |
ANC (/cmm) or Platelet count (/cmm) | <1000/ <1,00,000 on day 21- Postpone next chemo by 1 week |
|
|
|
| <1000/ <1,00,000 on day 28- Delay till recovery, and give reduced dose for next cycle | Give 80% of dose | Give 80% of dose |
|
Peripheral neuropathy | 3/ 4 Grade |
|
| Omit |
Ilius |
|
|
| Omit |
Bilirubin(mg/dL) | 2-3 |
| Give 75% of dose | Give 1mg/m2 |
| 3-4 |
| Give 50% of dose | Omit |
| 4-5 |
| Give 25% of dose | Omit |
| >5 |
| Omit | Omit |
HYPER-CVAD PART- B
Frequency: 21 days
Protocol:
- Inj. Methotrexate 1000mg/m2- on Day 1
- 200mg/m2- in 100ml NS over 2 hrs
- 800mg/m2- in 500ml NS over 22 hrs
- Inj. Folinic acid- 15mg/m2- Start at 36hrs of starting methotrexate- Give 6 hrly for 6 doses/ till methotrexate levels are <0.1micromol/L
- Inj. Cytarabine- 3000mg/m2- in 500ml NS over 2 hrs- BD- On Day 2 and Day 3 (Total 4 doses)
- Inj. Methyl prednisolone- 50mg in 100ml NS over 30min- From Day 1 to Day 3 (3 days)
- Inj. G-CSF- mcg- SC- BD- From Day 4
- IntrathecalMethotrexate- 12mg- on Day 2
- Intrathecal Cytosine- 100mg- on Day 8
Dose adjustments:
|
| Methotrexate | Cytarabine | Folinic acid |
MTX Level (micromol/L) | >20- at the end of infusion |
|
| Increase dose to 50mg/m2 |
Age | >60yrs |
| Decrease dose and give 1000mg/m2 |
|
ANC (/cmm) or Platelet count (/cmm) | <1000/ <1,00,000 on day 21- Postpone next chemo by 1 week |
| Give 1000mg/m2 |
|
| <1000/ <1,00,000 on day 28 | Give 75% of dose | Give 66% of dose |
|
Pleural effusion/ ascites |
| Give 50% of dose |
|
|
Grade 3 or 4 mucositis |
| Give 75% of dose |
|
|
S. Creatinine (mg/dL) | 1.3-3 | Give 50% of dose | Give 2000mg/m2 |
|
| >3 | Give 25% of dose | Omit |
|
Response Criteria:
- Day 8 Steroid response:
- Good steroid response: Absolute blast count <1000/cmm
- Poor steroid response: Absolute blast count ≥ 1000/cmm
- Definition of CR (All should be met)-
- No circulating blasts or extramedullary disease (Lymphadenopathy, Splenomegaly, Skin lesions, testicular enlargement, CNS disease)
- ANC >1000/cmm
- Platelet count >1,00,000/cmm
- Bone marrow: Normal trilineage hematopoiesis with less than 5% blasts (In BFM 2002 protocol this called as M1 status)
- M2 Status: 5-24% blasts
- M3 Status: ≥25% blasts
(Subject all M2 and M3 bone marrows for flow cytometry to rule out hematogones)
If no blasts due to hemorrhagic tap or marrow aplasia- Repeat BMA after 1 week without any intervening therapy
About Each Phase of Treatment:
- Induction therapy
- Given to reduce tumor burden by clearing as many leukemic cells as possible from bone marrow
- Protocol contains administration of steroids, anthracycline, Vincristine and L Asparginase in Phase 1. Cyclophosphamide and cytarabine in phase 2.
- Although Dexamethasone has high CNS penetration, there is high risk of infection and mortality with dexamethasone.
- Goal of induction is to achieve remission.
- Flow cytoemtry MRD is defined as <0.01% tumor cells of all nucleated marrow cells.
- Even in CR, patients have as many as 10 billion tumor cells.
- Failure to achieve remission occurs in 20% adult ALL and <3% in childhood ALL
- Mortality during induction- <3% in children and 20% in adults
- Consolidation therapy
- It is given to eliminate residual leukemic cells that remain after induction therapy
- It is started once normal hematopoiesis id restored
- It generally includes High dose methotrexate or High dose cytarabine with Etoposide
- Stem cell transplantation (Allogeneic or Autologous) is another method of consolidation
- Reinduction
- Given to remove any tumor cells which might have survived after consolidation
- Involves administration of same category chemotherapy agents which are given during induction
- Maintenance:
- Highest risk of relapse is during first 2-3years after completion of treatment
- Maintenance is given to prevent this relapse of disease as it eliminates minimal residual disease
- It includes daily administration of 6 MP and weekly administration of methotrexate.
- Periodic intrathecal chemotherapy is given to prevent CNS relapse
- It is continued for 2-3 years.
Targeted therapies used in the management of ALL
- Tyrosine kinase inhibitors: For BCR-ABL positive patients
- Imatinib- 600mg-OD
- Dasatinib
- Rituximab:
- For CD 20 positive B-ALL.
- Adding Rituximab led to improvement in overall 3-year survival to 75%, compared to 47% in historical controls.
- Inotuzumab
- CD22 directed antibody drug conjugate
- 21- 28 days cycle
- 1st cycle: Day 1: 0.8 mg/m², Day 8 and 15: 0.5 mg/m²
- Delay next cycles until ANC- >1000/cmm, Platelet count- >50,000/cmm. Further dose adjustments are necessary if recovery takes more than 7 days.
- If no CR/CRi may continue for up to 3 cycles. If no CR even after 3 cycles- Discontinue
- If CR/CRi- Decrease dose to: Day 1, 8, and 15: 0.5 mg/m² IV
- Permanently discontinue if there is VOD of liver or life threatening allergic reactions.
- Premedicate with hydrocortisone, paracetamol and avil.
- Administered in 50ml NS over 1hr.
- Observe patient during and for at least 1 hr post infusion.
- Prior to initial dose, do cytoreduction with a combination of hydroxyurea, steroids, and/or vincristine to achieve a peripheral blast count ≤10,000/mm³
- Patients proceeding to HSCT- Give 2 cycles
- Patients not proceeding to HSCT: Additional cycles may be administered; not to exceed 6 cycles
- Blinatumomab
- Bispecific CD19 directed CD3 T-cell engager
- 28 days cycle
- Premedicate with Inj. Dexamethasone- 10-20mg-IV
- <45Kg
- Induction: Cycle 1:
- Days 1-7: 5 mcg/m2/day continuous IV infusion (not to exceed 9 mcg/day), THEN
- Days 8-28: 15 mcg/m2/day (not to exceed 28 mcg/day)
- Days 29-42: Treatment-free interval
- Induction: Cycle 2:
- Days 1-28: 15 mcg/m2/day continuous IV infusion (not to exceed 28 mcg/day)
- Days 29-42: Treatment-free interval
- Consolidation: Cycles 3-5
- Days 1-28: 15 mcg/m2/day continuous IV infusion (not to exceed 28 mcg/day), THEN
- Days 29-42: Treatment-free interval
- Continued therapy: Cycles 6-9
- Days 1-28: 15 mcg/m2/day continuous IV infusion (not to exceed 28 mcg/day), THEN
- Days 29-84: Treatment-free interval
- Induction: Cycle 1:
- >45Kg (Fixed dose)
- Induction Cycle 1:
- Days 1-7: 9 mcg/day continuous IV infusion, THEN
- Days 8-28: 28 mcg/day
- Days 29-42: Treatment-free interval
- Induction Cycle 2:
- Days 1-28: 28 mcg/day continuous IV infusion, THEN
- Days 29-42: Treatment-free interval
- Consolidation (Cycle 3-5)
- Days 1-28: 28 mcg/day continuous IV infusion
- Days 29-42: Treatment-free interval
- Continued therapy (Cycles 6-9)
- Days 1-28: 28 mcg/day continuous IV infusion
- Days 29-84: Treatment-free interval
- Induction Cycle 1:
- For Mild to moderate cytokine release syndrome: Inj. Dexamethasone 5mg/m2- IV/PO- TID for 3 days and then taper over 4 days. Restart in lower dose and then gradually increase the dose.
- For severe cytokine release syndrome (Fever, headache, nausea, hypotension): Dexamethasone as above and permanently discontinue Blinatumomab.
- If seizures/ speech disorder/ confusion/ altered conciousness or allergic reactions develop- Permanently discontinue
- Common side effects: Infusion reactions, fever, cytopenia, edema
- Several other MAbs are being/ have been used, such as Epratuzumab (CD22), Alemtuzumab (CD52). But they are not being routinely used in clinical practice.
CNS directed therapy
- Prophylactic intrathecal chemotherapy has to be given as per the protocol throughout the treatment
- Cranial irradiation is given to
- All T-ALL patients
- CNS Positive at diagnosis
- Ph+ve cases
- Dose:
- Therapeutic- 1-2 years-12Gy and >2 years-18Gy
- Prophylactic- 12Gy for all
- RT should not be given to
- Infants <1 year of age and <2yrs who are planned for BMT.
- Patients with serious non-leukemic CNS disorders such as post MTX chronic leukoencephalopathy, h/o CVT/intracranial hemorrhage etc.
- Patients with chromosomal instability syndromes
- Technique of RT
- High energy conditions (6MV) with linear accelerator/ telecobalt-60 device
- Target volume must include all intracranial structures, both retrobulbar spaces, entire base of skull, and upper 2 segments of cervical spine. (Avoid shielding of eyes)
- Use custom made blocks/filters
- Daily dose per fraction- 1.5Gy- Delivered 5 sessions per week, until total target dose is reached.
- RT Can be given along with maintenance
- Side effects of RT
- Headache- Treated with Dexamethasone- 15mg/m2/day
- Apathy/ somnolence- 4-6 weeks after RT. Treatment includes avoiding physical/psychic overload, adequate sleep, recreation and protection from direct sunlight
- Development of brain tumors
- High dose methotrexate also provides significant protection against CNS relapse.
- If CNS positive at diagnosis (Status 3)-
- Give Intrathecal chemotherapy every week during phase 1 of induction
- Methotrexate - 5gm/m2
- Cranial irradiation after re-induction
- More number of intrathecal chemotherapies during maintenance
Management of testicular involvement
- Recheck testes on day 33- If completely regressed, continue with assigned therapy
- If there is space occupying lesion/ infiltration, do testicular biopsy.
- If biopsy shows viable tumour cells, treat as high-risk disease.
Management of mediastinal involvement
- Measure width of mediastinum in chest X ray at body of T5 vertebra at baseline and at day 33
- If tumour has completely disappeared by day 33, continue assigned therapy
- If not regressed, continue with phase 2 of induction. One week after completion of induction, do CT chest. If there is residual tissue, resect the residual tissue. If histopathology examination shows viable blasts, treat as high-risk disease. (If all this is not possible, do PET CT to see if there is residual disease. If PET is positive, supplemental therapy with radiation needs to be given)
Stem cell transplant in ALL
- Best results when done in CR1
- Higher cure rates, but has problem of GVHD
- GvL effect actually cures the disease
- Conditioning with TBI or Busulfan, along with cyclophosphamide or Etoposide
- Reduced intensity conditioning can be used in elderly patients
- Source of stem cells- Preferably unmanipulated bone marrow
- GVHD prophylaxis- Cyclosporine alone, to decrease incidence of relapse. (If MUD give Methotrexate as well)
- Haplo identical transplants to be done in highly specialized centres and in the context of a clinical trial
Supportive care (Refer to supportive care section)
- Management of tumour lysis syndrome
- Treatment of infections
- Transfusion therapy
- Management of chemotherapy side effects
Monitoring After Treatment/ Follow-up:
- Once in 3 months- History, examination and CBC for 3 years
Vaccination:
- Live vaccines are contraindicated during and up to 6 months after end of chemotherapy.
- Non-live vaccines are also best given after 6 months from the end of treatment for durable immunity.
- Annual inactivated influenza vaccine is the only vaccine recommended for all children during chemotherapy whereas hepatitis B vaccine is recommended only for previously unimmunised children with risk of transfusion associated transmission of infection.
- Sibling immunization should continue uninterrupted except for oral polio vaccine which needs to be substituted by the injectable vaccine.
- Inactivated influenza vaccine is recommended and varicella vaccine is encouraged for all contacts including siblings.
For detailed guidelines: Click Here
Late effects:
- Osteonecrosis and osteoporosis due to steroids
- Cognitive impairment
- Cardiomyopathy due to anthracyclines
Specific types of acute lymphoblastic leukemia
- B-lymphoblastic leukaemia/lymphoma with high hyperdiploidy
- B-ALL with a karyotype comprising 51-65 chromosomes, characterized by recurrent, non-random gains of one or more copies of entire chromosomes, usually chromosomes X, 4, 6, 10, 14, 17, 18 and 21 in the absence of type-defining gene fusions and rearrangements.
- Incidence:
- Children- 25-35% of B-ALL cases
- Adults- 7-8% of B-ALL cases
- Prognosis: Vary favourable (Overall survival >90% in children)
- B-lymphoblastic leukaemia/lymphoma with hypodiploidy
- B-ALL with presence of less than or equal to 43 chromosomes
- Subtypes:
- Near haploid: 24-31 chromosomes
- Low- hypodiploid: 32-39 chromosomes
- High- hypodiploid: 40-43 chromosomes
- Incidence:
- Children- <1% of B-ALL cases
- Adolescents- 5% of B-ALL cases
- Adults- >10% of B-ALL cases
- Most of the children have germline p53 mutations
- Prognosis is poor
- B-lymphoblastic leukaemia/lymphoma with iAMP21
- Intrachromosomal amplification of chromosome 21 ( iAMP21) is due to gains and gross rearrangements involving the long arm of chromosome 21
- Accounts for 2% of paediatric B-ALL cases
- Seen mostly in people with constitutional Robertsonian translocation between chromosomes 15 and 21
- FISH for RUNX1 shows additional copies of the RUNX1 signals (Usually ≥5)
- Prognosis: High risk of relapse
- B-lymphoblastic leukaemia/lymphoma with BCR::ABL1 fusion
- Seen in 3% of childhood ALL and 25% of adult ALL
- 80-90% have 190kDa fusion protein (p190; transcript e1a2)
- CML in B lymphoid blast crisis usually shows p210 fusion protein
- Positive for CD19, CD10, CD45 and frequently myeloid markers
- Additional chromosomal abnormalities include: Gain of a Ph chromosome, monosomy 7, +8, +X, +21 and del(9p)
- Mutation of IKZF1, PAX5, CDKN2A and CDKN2B are commonly seen
- Traditionally associated with poor prognosis but now improved survival due to availability of targeted therapy (TKI).
- Patients achieving IS of <0.01% have favourable prognosis.
- IKZF1 alterations are associated with poor response to TKIs and worse overall survival.
- B-lymphoblastic leukaemia/lymphoma with BCR::ABL1-like features
- B-ALL with DNA alterations that induce a phenotype similar to that of BCR::ABL1+ B-ALL, but lack the pathognomonic BCR::ABL1 rearrangement
- Accounts for 10–15% B-ALL in children and 25-30% B-ALL in adults
- These patients have mutations that result in activation of JAK/STAT, ABL-class or other kinase signalling pathways, which include:
- Rearrangements of CRLF2 with IGH or P2RY8
- JAK2 gene fusions
- EPOR Rearrangements
- Alterations in SH2B3, IL2RB, and TYK2 genes
- Flow cytometry: Over-expression of TSLPR which is a surrogate marker of CRLF2 rearrangement
- Whole transcriptome analysis: Ideally needed to define this entity, however this test is not routinely available.
- Prognosis: Poor response to therapy and poor overall survival
- Promising outcomes with the addition of tyrosine kinase inhibitors directed against specific genomic lesions
- B lymphoblastic leukaemia/lymphoma with KMT2A (MLL) rearrangement
- Accounts for 70-80% of leukaemia in infants aged <1 year
- Less common in older children, but incidence increases with advancing age.
- In case of patients <1year, KMT2A rearrangement is acquired in utero.
- More than 90 gene partners for KMT2A (MLL) have been identified. Most common is t(4;11) (q21;q23)- MLL- AF4, Next most common are t(19:11)- MLL-ENL and t (9:11)- MLL-AF9
- These rearrangements can be identified by FISH/ RT-PCR/ NGS.
- MLL normally maintains expression of specific Hox genes by binding to DNA & recruiting histoneacetylase that keeps the chromatin in an open conformation accessible to transcriptional activators.
- MLL interacts with antiphophatase SBF1, which positively regulates kinase signalling pathways
- Associated with large leukemic cell burden and frequent CNS involvement
- CD 10 negative and aberrant CD15 positive B – cell ALL. Often positive for myeloid markers.
- Poor prognosis
- B lymphoblastic leukaemia/lymphoma with ETV6::RUNX1 fusion
- Associated with t(12;21) (p13;q22)
- Most common genetic rearrangement in childhood ALL (25%)
- Cryptic translocation. Requires FISH/ PCR/ RNA sequencing for detection
- Positive for CD10, CD19 and CD34
- Associated with good prognosis
- Associated with increased sensitivity to Asparaginase
- ETV6::RUNX1 fusion protein represses AML-1 mediated transcriptional activation through a dominant negative mechanism.
- Normally AML1 binds to DNA as heterodimer with CBFB & is essential for development of definitive haematopoiesis
- B-lymphoblastic leukaemia/lymphoma with ETV6::RUNX1-like features
- Has a gene expression profile similar to B-ALL with ETV6::RUNX1 in absence of the ETV6::RUNX1 translocation
- Prognosis: Not clear
- B lymphoblastic leukaemia/lymphoma with TCF3::PBX1 fusion
- Associated with t(1;19)
- Fusion protein induces cell differentiation arrest
- Seen in 5 – 6% of childhood ALL especially in pre B ALL
- No significant association with response to therapy
- B lymphoblastic leukaemia/lymphoma with IGH::IL3 fusion
- Associated with t (5:14) (q31; q32)
- Associated with eosinophilia driven by IL3
- Blasts may be <20% in BM and undetectable in peripheral blood
- B lymphoblastic leukaemia/lymphoma with TCF3::HLF fusion
- Associated with t(17;19)
- Seen in 0.5 – 1% of childhood ALL
- This fusion protein inhibits apoptosis
- Associated with hypercalcemia, DIC
- Prognosis is poor
- B-lymphoblastic leukaemia/lymphoma with other defined genetic alterations:
- This category includes B-ALL with following genetic alterations:
- DUX4 rearrangement
- MEF2D rearrangement
- ZNF384 rearrangement
- PAX5 alteration
- PAX5 p.P80R
- NUTM1 rearrangement
- MYC rearrangement
- This category includes B-ALL with following genetic alterations:
- B-lymphoblastic leukaemia/lymphoma, NOS
- It includes B-ALL/LBL cases that do not meet the criteria for any B-ALL/LBL types that are defined by defined genetic abnormalities.
- Accounts for 5-10% of all B-ALL/LBL cases
- Before diagnosis, other subtypes must be excluded through cytogenetic analysis (karyotype, FISH, chromosomal microarray), genetic sequencing (RNA, DNA) and gene expression profiling.
- T – Acute Lymphoblastic leukaemia / Lymphoma- NOS
- Account for 15% of childhood and 25% of adult ALL.
- Male predominance
- High circulating leucocyte counts. Morphologically blasts are indistinguishable from those of B-ALL.
- Flow cytometry: Described above
- Genetic defects are seen at 11q, 7q and 7p commonly
- High risk of CNS involvement
- Mediastinal (thymus), hepatosplenomegaly and lymph node involvement is common.
- Radiographically evident thymic mass
- No robust clinical and genetic prognostic markers are available
- Earlier had poor treatment outcome compared to B-ALL, but with intensification therapy, this gap has narrowed.
- Early T cell precursor Lymphoblastic leukaemia / Lymphoma (ETP-ALL)
- Composed of blasts committed to the T-cell lineage with a unique immunophenotype that includes expression of stem cell markers and/or myeloid lineage markers.
- Account for 12-17% of pediatric T-ALL and 22-40% of adult T-ALL.
- It was recognised as a distinct type of T-ALL, based on gene expression signatures.
- Criteria for diagnosis: Immunophenotypically, must meet all 5 criteria for antigen expression:
- CD3 (cytoplasmic + and surface -)
- Absent myeloperoxidase (<10% by flow cytometry, <3% by cytochemistry)
- Absent CD1a and CD8
- ≥25% of blasts with ≥1 of stem cell or myeloid markers: CD34, CD117, CD13, CD33, CD65, CD11b, HLA-DR
- Dim to negative CD5 (<75% of blasts positive)
- If first 4 criteria are met but >75% blasts are positive for CD5, then it is called “Near-ETP-ALL”.
- Poor prognosis
Figures:
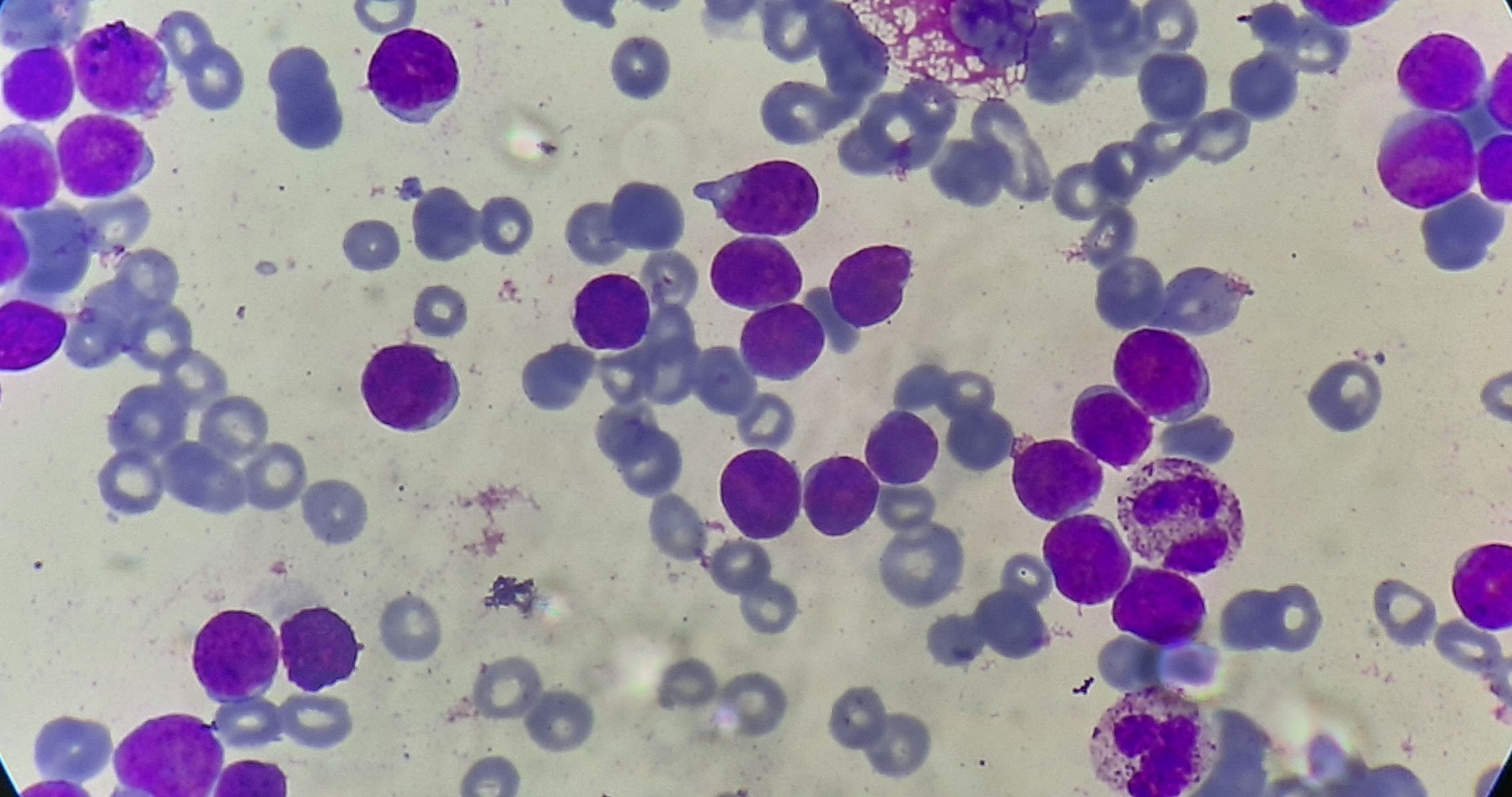
Figure 4.1.1- Acute lymphoblastic leukemia- L1
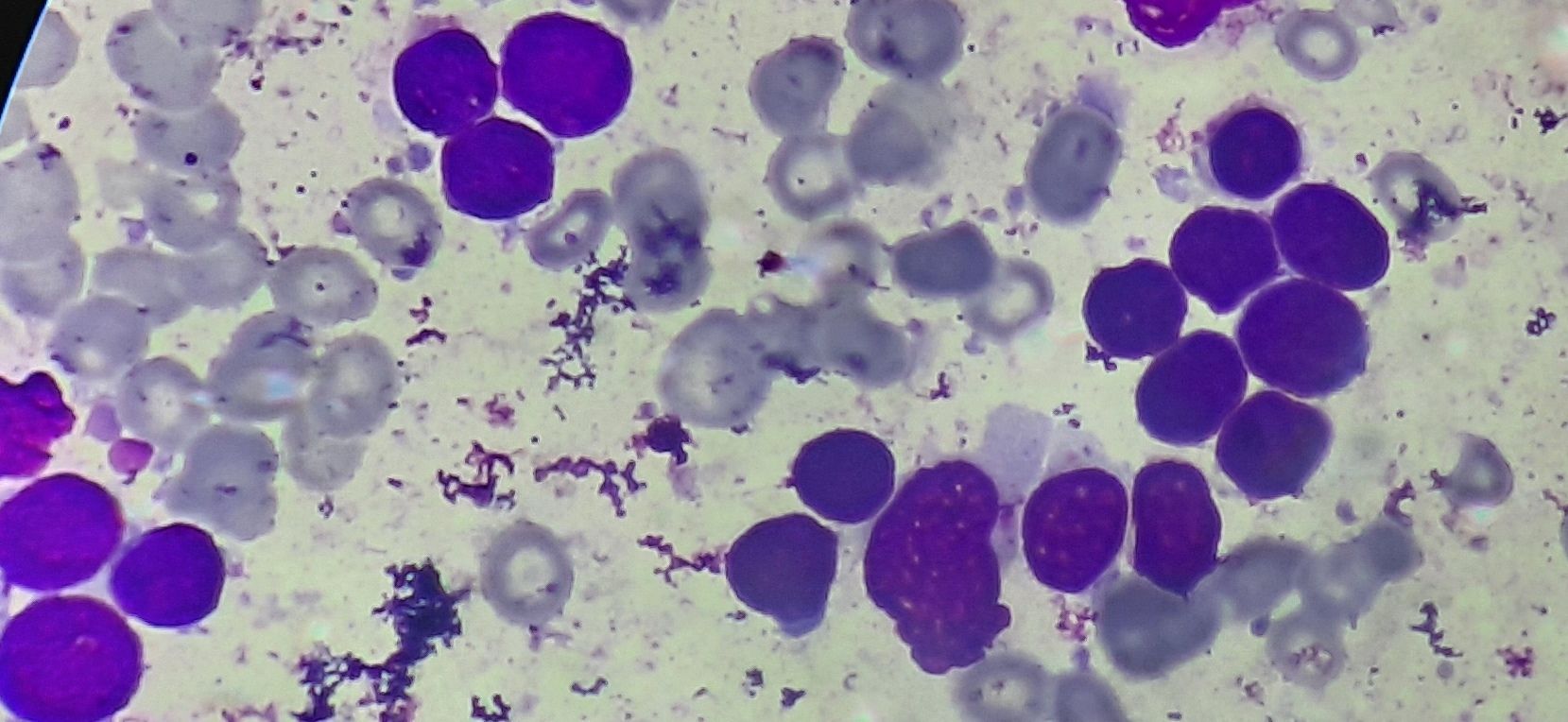
Figure 4.1.2- Acute lymphoblastic leukemia- L2
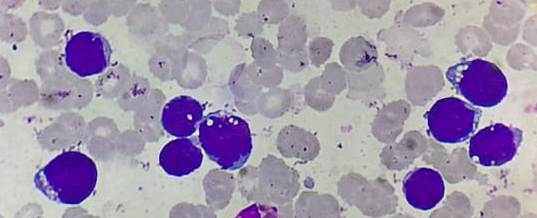
Figure 4.1.3- Acute lymphoblastic leukemia- L3
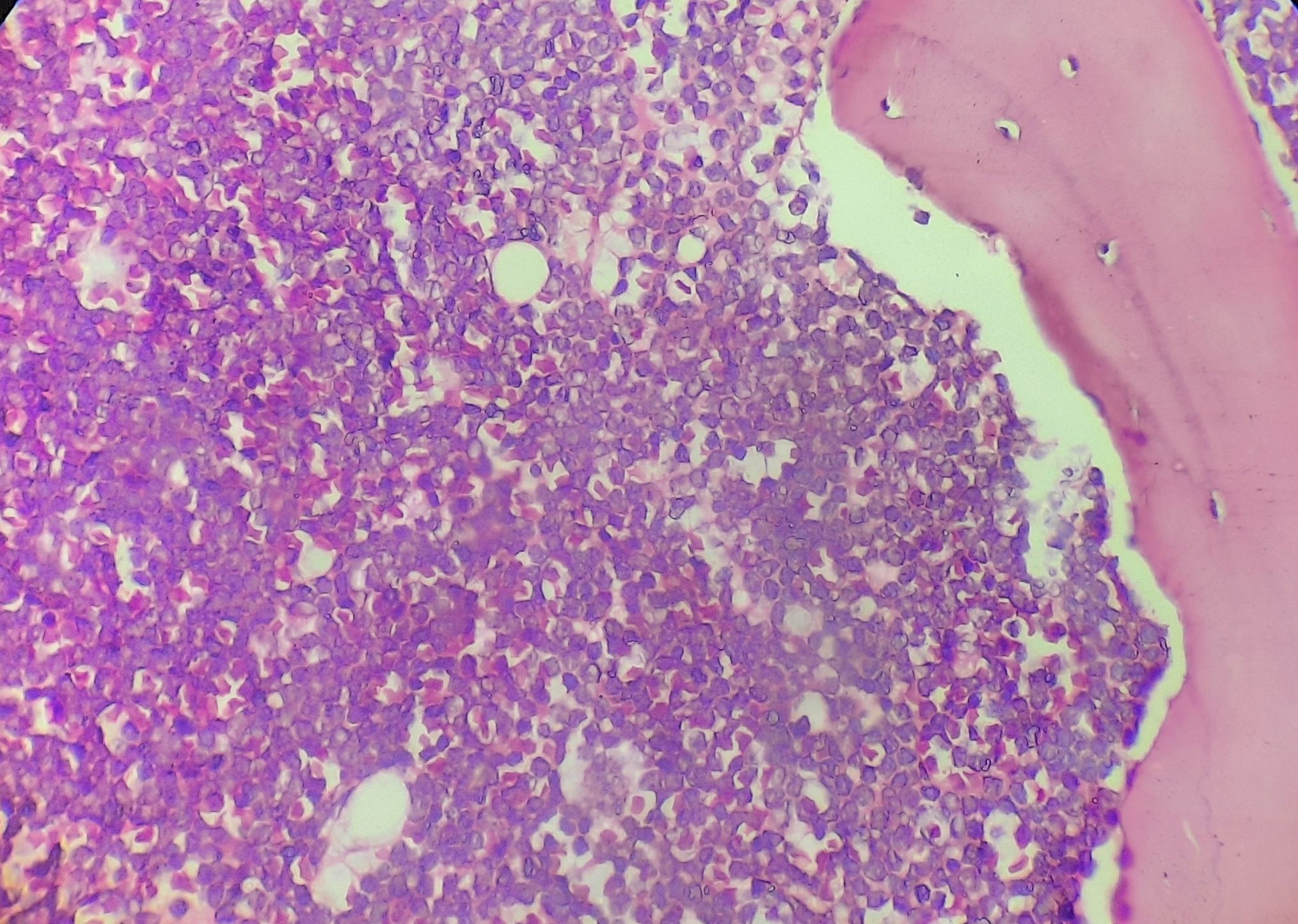
Figure 4.1.4- Acute lymphoblastic leukemia- Bone marrow biopsy
Recent advances:
Donor-derived multiple leukemia antigen–specific T-cell therapy to prevent relapse after transplant in patients with ALL
In a recent study, researchers infused antigen–specific T cells (mLSTs) targeting PRAME, WT1, and survivin antigens to patients who had undergone allo SCT for ALL. The goal was to enhance GVL effect without increasing the risk of GVHD. 6 of 8 patients who received this therapy remained in long-term complete remission.
doi.org/10.1182/blood.2021014648
Blinatumomab Followed by POMP Maintenance in Older Patients with Ph- ALL- SWOG 1318 study
In a recent study by Anjali Advani et al, blinatumomab as induction and consolidation therapy followed by prednisone, vincristine, 6-mercaptopurine, and methotrexate (POMP) maintenance was evaluated in elderly Ph- ALL patients. 19 out of 29 patients achieved CR. 3-year disease free survival was 37%.
doi.org/10.1200/JCO.21.01766
Efficacy and safety of CD19-specific CAR T cell–based therapy in B-cell acute lymphoblastic leukemia patients with CNS leukemia
The present study included 48 patients with relapsed/refractory B-ALL with CNS leukemia. The infusion resulted in an overall response rate of 87.5% in bone marrow (BM) disease and remission rate of 85.4% in CNSleukemia. Study proved that CD19 CAR T-cell therapy may provide a potential treatment option for previously excluded patients with CNS leukemia, with manageable neurotoxicity.
https://doi.org/10.1182/blood.2021013733
Bortezomib in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia
Bortezomib was examined in the Children's Oncology Group phase III clinical trial AALL1231in newly diagnosed T-ALL. Children and young adults with T-ALL/T-LL were randomly assigned to a modified augmented Berlin-Frankfurt-Münster chemotherapy regimen with/without bortezomib during induction and delayed intensification. Patients with T-LL had significantly improved EFS and OS with bortezomib on the AALL1231 backbone. Systemic therapy intensification allowed elimination of CRT in more than 90% of patients with T-ALL without excess relapse.
https://doi.org/10.1200/JCO.21.02678
The role of allogeneic transplant for adult Ph+ ALL in CR1 with complete molecular remission
Philadelphia chromosome-positive acute lymphoblastic leukemia has been associated with poor outcomes, and allogeneic hematopoietic cell transplantation (allo-HCT) is recommended in first complete remission. This study compared outcomes of those who did and did not receive allo-HCT in first remission. The allo-HCT cohort was younger with better performance status. On multivariable analysis, allo-HCT was not associated with improved overall survivalor relapse-free survival compared with non-HCT treatment. In conclusion, adult patients with Ph+ ALL who achieved CMR within 90 days of starting treatment did not derive a survival benefit from allo-HCT in CR1 in this retrospective study.
https://doi.org/10.1182/blood.2022016194
Obesity as a predictor of treatment-related toxicity in children with acute lymphoblastic leukaemia
This study included 1443 children aged 2·0–17·9 years with ALL treated with the Nordic Society of Pediatric Haematology and Oncology (NOPHO) ALL2008 non-high-risk protocol. Obese children aged ≥10 years had increased asparaginase-related toxicities compared with healthy-weight older children: thromboses and anaphylactic reactions. The high prevalence of toxicity and a higher risk of truncation of asparaginase may play a role in the poor prognosis of obese children aged ≥10 years with ALL.
https://doi.org/10.1111/bjh.17936
Consolidation Therapy with Blinatumomab B Acute Lymphoblastic Leukemia: Results from the ECOG-ACRIN E1910 Randomized Phase III
National Cooperative Clinical Trials Network Trial randomizing patients to conventional chemotherapy with or without blin to determine if patients who become MRD negative (<0.01%) after induction chemotherapy (chemo) can have improved outcomes with the addition of blin.Study showed tthat addition of blin to consolidation chemo resulted in a significantly better overall survival in pts with newly diagnosed B-lineage ALL who were MRD negative after intensification chemo. No significant safety concerns were noted.
https://doi.org/10.1182/blood-2022-171751
EBV-driven lymphoid neoplasms associated with pediatric ALL maintenance therapy
The development of a second malignancy after the diagnosis of childhood acute lymphoblastic leukemia is a rare event. Present study showed that mature B-cell lymphoproliferations was the dominant subtype (56 of 85 cases). The majority exhibited histopathological characteristics associated with immunodeficiency (65%), predominantly evidence of Epstein-Barr virus–driven lymphoproliferation.
https://doi.org/10.1182/blood.2022016975
Coadministration of CD19- and CD22-Directed Chimeric Antigen Receptor T-Cell Therapy in Childhood B-Cell Acute Lymphoblastic Leukemia
Complete remission was achieved in 99.0% of the 194 patients with refractory leukemia or hematologic relapse, all negative for minimal residual disease. Their overall 12-month event-free survival was 73.5%. Consolidative transplantation and persistent B-cell aplasia at 6 months were associated with favorable outcomes.
https://doi.org/10.1200/JCO.22.01214
Tisagenlecleucel in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia
In ELIANA trial, tisagenlecleucel provided an overall remission rate of 81% in pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia. This is a 3 year follow up update of same 79 pediatric and young adult patients. The overall remission rate was 82%. Event-free survival was 44% and overall survival was 63% at 3 years overall.
https://doi.org/10.1200/JCO.22.00642
VANDA regimen followed by blinatumomab in B-precursor acute lymphoblastic leukaemia with first relapse
Present study aimed to assess the activity of blinatumomab in concomitant association with intensive chemotherapy. Seventeen patients with R/R BCP-ALL were treated with combination of blinatumomab and VANDA (etoposide, cytarabine, mitoxantrone, dexamethasone and asparaginase) regimen. Complete remission was achieved in 14/17 patient (82%) and 11/17 were transplanted. One-year leukaemia-free survival was 58.8% for the whole cohort and 90.9% for transplanted patients.
https://doi.org/10.1111/bjh.18218
Extended vincristine and dexamethasone pulse therapy may not be necessary for children with TCF3-PBX1 positive acute lymphoblastic leukaemia
Present non-inferiority analysis included 263 newly diagnosed TCF3-PBX1 positive ALL children who were stratified and randomly assigned (1:1) to receive seven additional VD pulses (the control group) or not (the experimental group) in the CCCG-ALL-2015 clinical trial from January 2015 to December 2019. With a median follow-up of 4.2 years, the 5-year event-free survival (EFS) and 5-year overall survival (OS) in the control group were 90.1% and 94.7% comparable to those in the experimental group 89.2% and 95.6% respectively.
https://doi.org/10.1111/bjh.18437
Central nervous system status is prognostic in T-cell acute lymphoblastic leukemia: a Children’s Oncology Group report
This analysis examined the prognostic significance of central nervous system (CNS) leukemic involvement in newly diagnosed T-cell acute lymphoblastic leukemia (T-ALL) using data from two clinical trials. The study found that patients with CNS-1 and CNS-2 status had similar outcomes, regardless of cranial radiation therapy (CRT) or intensified corticosteroids. However, patients with CNS-3 status had inferior event-free survival (EFS) and overall survival (OS), and the use of nelarabine improved outcomes for this group. Novel approaches are needed to address the challenges associated with CNS-3 status in T-ALL.
https://doi.org/10.1182/blood.2022018653
Outcomes after nonresponse and relapse post-tisagenlecleucel in patients with B-ALL
ELIANA trial demonstrated nonresponse and relapse rates of 14.5% and 28%, respectively after CAR-T cell therapy. Present study aimed to establish survival outcomes after nonresponse and both CD19+ and CD19– relapses and explored treatment variables associated with inferior survival. The overall survival (OS) at 12 months was 19% across nonresponders. Ninety-five percent of patients with nonresponse had high preinfusion disease burden. CD19– relapse was associated with significantly decreased OS as compared with patients who relapsed with conserved CD19 expression.
https://doi.org/10.1200/JCO.22.01076
Busulfan Plus Cyclophosphamide Versus Total Body Irradiation Plus Cyclophosphamide for B-ALL
Present study investigated the efficacy and toxicity of busulfan plus cyclophosphamide (BuCy) and TBI plus cyclophosphamide (TBI-Cy) conditioning in allo-HSCT for adult standard-risk B-cell-ALL in first complete remission (CR1). The 2-year overall survival was 76.6% and 79.4%, indicating noninferiority of BuCy. The 2-year relapse was 20.2% and 18.4%, and the nonrelapse mortality was 11.0% in both groups. There were no differences in regimen-related toxicity, graft-versus-host disease, or late effects between the two groups.
https://doi.org/10.1200/JCO.22.00767
Venous thromboembolism incidence associated with pegylatedasparaginase (ASP) compared to the native L-ASP
This retrospective cohort study was conducted to assess the incidence of venous thromboembolism (VTE) in adult patients with acute lymphoblastic leukemia (ALL) receiving different forms of asparaginase (ASP) chemotherapy. The study compared patients who received native L-ASP (175 patients, 2011-2019) with those who received pegylated (PEG)-ASP (70 patients, 2018-2021) due to the unavailability of native L-ASP in Canada since 2019. During the Induction phase, 10.29% of patients on L-ASP developed VTE, while 28.57% of patients on PEG-ASP developed VTE. Similarly, during the Intensification phase, the incidence of VTE was 13.64% in the L-ASP group and 34.37% in the PEG-ASP group. Statistical analysis confirmed that PEG-ASP was associated with a higher incidence of VTE compared to L-ASP, even with prophylactic anticoagulation.
https://doi.org/10.1111/bjh.18683
Impact of asparaginase discontinuation on outcomes of children with acute lymphoblastic leukaemia
The Japan Association of Childhood Leukemia Study implemented the ALL-02 protocol, which included additional chemotherapies and intensified corticosteroid administration to compensate for the discontinuation of asparaginase. The study included 1192 patients, and the discontinuation rate for asparaginase was 7.4%, with a significant decrease in discontinuation due to allergies compared to previous protocols. Discontinuation of asparaginase had a negative impact on event-free survival (EFS) for patients with T-ALL and high-risk B-cell ALL, particularly when discontinued before maintenance therapy. The study found that additional chemotherapies were not able to fully compensate for the discontinuation of asparaginase, highlighting the challenge of finding suitable alternatives. However, intensive corticosteroid treatment showed potential in reducing asparaginase allergy. The results of this study contribute to the optimization of asparaginase use in ALL treatment.
https://doi.org/10.1111/bjh.18745
Impact of vincristine-steroid pulses during maintenance for B-cell pediatric ALL
This systematic review and meta-analysis examined the impact of reducing the frequency of vincristine-steroid pulses in maintenance therapy for pediatric B-cell acute lymphoblastic leukemia (ALL). The analysis included 25 publications and 12,513 patients. The results suggest that there is no significant benefit in event-free survival between more frequent or less frequent pulses in contemporary trials, in contrast to historical trials. However, reducing pulse frequency is associated with increased odds of grade 3+ nonhepatic toxicity.
https://doi.org/10.1182/blood.2022018899
Dasatinib with intensive chemotherapy in de novo paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia
This study investigated the efficacy of dasatinib, a second-generation ABL-class inhibitor, combined with intensive chemotherapy in children with newly diagnosed Philadelphia chromosome-positive (Ph-positive) acute lymphoblastic leukemia (ALL). The study enrolled 106 eligible patients who received dasatinib plus chemotherapy, with 82% classified as standard risk and 18% as high risk. The primary endpoint, 3-year event-free survival, was superior to chemotherapy alone and non-inferior to imatinib plus chemotherapy. The most frequent adverse events were febrile neutropenia and bacteraemia, and there were no deaths related to dasatinib. Overall, dasatinib plus chemotherapy demonstrated safety and efficacy, achieving similar event-free survival compared to previous Ph-positive ALL trials, even with limited use of hematopoietic stem-cell transplantation in first complete remission.
https://doi.org/10.1016/S2352-3026(23)00088-1
A novel, oncogenic and targetable SEPTIN6::ABL2 fusion in T-ALL
The study highlights the presence of the SEPTIN6::ABL2 fusion as an oncogenic driver in T-ALL and demonstrates its sensitivity to TKIs. The findings suggest that targeted therapies using TKIs could be a promising approach for treating T-ALL patients with this fusion, potentially offering a more effective treatment option for this subgroup of patients. This work underscores the importance of identifying and characterizing novel genetic alterations to develop precision therapies for aggressive malignancies like T-ALL.
https://doi.org/10.1111/bjh.18901
Rituximab in pediatric acute lymphoblastic leukemia
This study investigated the impact of rituximab administration during induction therapy for pediatric patients with B-cell acute lymphoblastic leukemia (B-ALL), specifically looking at its effect on pegaspargase allergies and antibody production. Rituximab was hypothesized to reduce allergic reactions to pegaspargase by decreasing antibody-producing CD20-positive B cells. Patients were randomized to receive rituximab or not during different timeframes. While rituximab recipients experienced a high rate of infusion reactions, there were no significant differences in the incidence of pegaspargase reactions, anti-pegaspargase antibodies, or minimal residual disease levels. CD20 expression was lower in rituximab recipients, but it did not translate to reduced allergic reactions or antibody production related to pegaspargase.
https://doi.org/10.1038/s41375-023-01992-z
Improved Outcome for ALL by Prolonging Therapy for IKZF1 Deletion
This study presents the ALL11 protocol, which improved outcomes for pediatric acute lymphoblastic leukemia (ALL) patients by stratifying therapy based on minimal residual disease-defined risk groups. Results were compared to the ALL10 protocol. ALL11 showed a 5-year overall survival (OS) of 94.2%, event-free survival (EFS) of 89.0%, and low cumulative risk of relapse (CIR) at 8.2%. Prolonged maintenance therapy improved outcomes for patients with IKZF1-deleted ALL. Reduced therapy was effective for patients with ETV6::RUNX1, Down syndrome, and poor prednisone responders. These findings provide insights into tailored treatment strategies for different risk groups, although the study used historical controls.
https://doi.org/10.1200/JCO.22.02705
Orally bioavailable GSPT1/2 degrader SJ6986 in treatment of ALL
The novel cereblon modulator SJ6986 has been developed as a potential treatment for high-risk acute lymphoblastic leukemia (ALL). It targets GSPT1 and GSPT2 proteins for degradation, showing potent cytotoxicity against ALL cell lines. In vitro and in vivo tests demonstrated SJ6986's effectiveness in inducing apoptosis, disrupting cell cycle progression, and suppressing leukemic cell growth. It exhibited better outcomes compared to CC-90009, another GSPT1 degrader, due to its favorable pharmacokinetics and minimal impact on differentiation of normal cells. CRISPR/Cas9 screening confirmed the involvement of CRL4CRBN complex components in SJ6986's action, making it a promising candidate for clinical development.
https://doi.org/10.1182/blood.2022017813
Chemo-free regimen for Adult Ph-positive acute lymphoblastic leukaemia
This study conducted a phase 2 trial of dasatinib plus prednisone as a chemo-free regimen for newly diagnosed Ph+ ALL. Out of 41 enrolled patients, 95% achieved complete remission (CR), with 10 of them achieving a complete molecular response. The 2-year disease-free survival (DFS) was 100% for those receiving hematopoietic stem cell transplantation (HSCT) at CR1 and 33% for those receiving chemotherapy alone. Among young and elderly patients, the 2-year DFS was similar (51% vs. 45%) when censored at the time of HSCT. Allogeneic HSCT provided a survival advantage, and patients without HSCT had a 2-year overall survival of 45%. Marrow recurrences were observed in 12 patients. The study also found an association between IKZF1 gene deletion and relapse.
https://doi.org/10.1111/bjh.18975
Paediatric-inspired versus adult chemotherapy regimens on survival of high-risk Philadelphia-negative B-ALL
The PASS-ALL study compared the impact of pediatric-inspired versus adult chemotherapy regimens on survival in adolescents and young adults (AYA) with high-risk Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia (HR PH-ve B-cell ALL) eligible for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Among 143 enrolled patients from five centers, those in the pediatric-inspired cohort (n=77) demonstrated significantly better 3-year leukemia-free survival (LFS) at 72.2% compared to the adult protocol cohort (n=66) at 44.6%. Time-to-positive minimal residual disease (TTP-MRD) post-HSCT differed significantly between cohorts, with a lower cumulative relapse incidence observed in the pediatric-inspired group. Multivariate analysis identified the pediatric-inspired regimen as a predictive factor for LFS, indicating its potential to improve outcomes in HR B-cell PH-ve ALL patients undergoing allo-HSCT through deeper MRD response and reduced relapse rates.
https://doi.org/10.1111/bjh.19223
Pediatric leukemia and maternal occupational exposure to anticancer drugs
This cohort study investigated the association between parental occupational exposure to hazardous medical agents or ionizing radiation and the risk of childhood cancer in offspring. Data from a large birth cohort in Japan were analyzed, including 104,062 fetuses, with the primary outcome being the development of leukemia or brain tumors in the first 3 years after birth. Maternal exposure to anticancer drugs was found to be associated with an increased risk of leukemia in offspring older than 1 year, particularly acute lymphoblastic leukemia. These findings suggest a potential risk factor for childhood leukemia and highlight the importance of preventing maternal exposure to anticancer drugs to reduce the risk of childhood malignant neoplasms.
https://doi.org/10.1182/blood.2023021008
Blinatumomab for First-Line Treatment of Children and Young Persons With B-ALL
This study investigated the efficacy of blinatumomab (Blina) as a less toxic alternative to intensive chemotherapy in children and young persons (CYP) with B-cell acute lymphoblastic leukemia (B-ALL) who were intolerant or resistant to chemotherapy. Data from 105 patients showed that Blina was well tolerated, with minimal toxicity events, and resulted in a high response rate among patients with minimal residual disease pre-treatment. Patients receiving Blina had similar 2-year event-free survival and overall survival rates compared to matched controls receiving standard chemotherapy, suggesting that Blina is a safe and effective first-line treatment for chemotherapy-intolerant CYP with ALL.
https://doi.org/10.1200/JCO.23.01
CD7 targeted “off-the-shelf” CAR-T demonstrates robust in vivo expansion and high efficacy in the treatment of patients with relapsed and refractory T cell malignancies
GC027, an "off-the-shelf" allogeneic CD7-targeted CAR-T therapeutic product for T-cell acute lymphoblastic leukemia (T-ALL), has shown promising results in a study. In 11 out of 12 patients with relapsed and refractory T-ALL, GC027 led to rapid eradication of T-lymphoblasts, achieving complete response within one month after infusion. The CAR-T cells expanded quickly, reaching peak levels around 5-10 days post-infusion, and for most responders, GC027 was undetectable four weeks later. With a manageable toxicity profile, GC027 demonstrated superior clinical efficacy compared to standard chemotherapy regimens in treating relapsed and refractory T-cell malignancies.
https://doi.org/10.1038/s41375-023-02018-4
Prognostic significance of ETP phenotype and minimal residual disease in T-ALL
In a study involving 1256 newly diagnosed children and young adults with T-cell acute lymphoblastic leukemia (T-ALL) enrolled in COG AALL0434, the early thymic precursor (ETP) immunophenotype, near-ETP, and non-ETP groups showed excellent 5-year event-free survival (EFS) and overall survival (OS) rates, with no significant differences between the groups (ETP: 80.4% EFS, 86.8% OS; near-ETP: 81.1% EFS, 89.6% OS; non-ETP: 85.3% EFS, 90.0% OS). Induction failure rates were higher for ETP and near-ETP than non-ETP, but no differences in EFS or OS were observed when subjects were stratified by the early response to treatment, and persistent postinduction disease was associated with inferior outcomes regardless of the ETP phenotype.
https://doi.org/10.1182/blood.2023020678
Outcomes in Children, Adolescents, and Young Adults With Down Syndrome and ALL: A Report From the Children's Oncology Group
The study analyzed data from patients with Down syndrome (DS) and B-cell acute lymphoblastic leukemia (B-ALL) enrolled in Children's Oncology Group trials between 2003 and 2019. The analysis included 743 DS patients and 20,067 non-DS patients aged 1-30 years on four B-ALL standard-risk (SR) and high-risk trials. Patients with DS had higher rates of minimal residual disease (MRD) ≥0.01% at the end of induction. The 5-year event-free survival (EFS) and overall survival (OS) were significantly poorer for DS patients compared to non-DS patients. Multivariable analysis identified age >10 years, white blood count >50 × 10^3/μL, and end-induction MRD ≥0.01% as risk factors associated with inferior EFS in the DS cohort. DS patients demonstrated higher 5-year cumulative incidence of relapse, death in remission, and induction death. Treatment-related toxicities, including mucositis, infections, and hyperglycemia, were more frequent in DS patients.
https://doi.org/10.1200/JCO.23.00389
Inotuzumab Ozogamicin as Induction Therapy for Ph-Negative B-ALL
The INITIAL-1 trial evaluated inotuzumab ozogamicin and dexamethasone as induction therapy in 45 older patients (age >55) with newly diagnosed, CD22-positive, BCR::ABL-negative B-precursor acute lymphoblastic leukemia (B-ALL). All patients achieved complete remission, and 71% had no measurable residual disease after the third induction. After a median follow-up of 2.7 years, event-free survival at one and 3 years was 88% and 55%, while overall survival was 91% and 73%, respectively. The regimen was well-tolerated, indicating potential for integrating inotuzumab ozogamicin into first-line regimens for older B-ALL patients.
https://doi.org/10.1200/JCO.23.0054
IGJ and SPATS2L immunohistochemistry sensitively and specifically identify BCR::ABL1+ and BCR::ABL1-like B-acute lymphoblastic leukaemia
A study involving 118 B-acute lymphoblastic leukaemia (B-ALL) cases identified surrogates for BCR::ABL1-like B-ALL using immunohistochemistry (IHC). Immunoglobulin joining chain (IGJ) IHC showed 83% sensitivity, 95% specificity, 89% positive predictive value (PPV), and 90% negative predictive value (NPV). Spermatogenesis associated serine-rich 2-like (SPATS2L) staining had similar sensitivity and NPV but lower specificity (85%) and PPV (70%). Combining IGJ and SPATS2L increased sensitivity (93%) and NPV (95%). The findings suggest that IGJ and/or SPATS2L IHC could be used to identify BCR::ABL1-like B-ALL or select cases for confirmatory molecular/genetic testing, particularly in resource-limited settings.
https://doi.org/10.1111/bjh.19142
An “off-the-shelf” CD2 universal CAR-T therapy for T-cell malignancies
In addressing the challenges of T-cell malignancies, a novel allogeneic "universal" CD2-targeting CAR-T cell (UCART2) was developed, demonstrating efficacy against T-ALL and CTCL in preclinical models. CD2 deletion in UCART2 prevented fratricide and T-cell receptor removal prevented graft-versus-host disease (GvHD). Additionally, investigating the impact of CD2 on CAR-T function, CD2 deletion in UCART19 reduced effector cytokine frequencies, resulting in reduced anti-tumor efficacy. However, combining CD2-deleted UCART19 with rhIL-7-hyFc, a long-acting recombinant human interleukin-7, reversed the reduced efficacy, suggesting a potential approach for treating T-cell malignancies.
https://doi.org/10.1038/s41375-023-02039-z
Dasatinib-Blinatumomab Protocol for Adult Philadelphia-Positive ALL
The long-term results of the frontline trial (GIMEMA LAL2116, D-ALBA) for adult Philadelphia-positive ALL (Ph+ ALL) involving dasatinib and blinatumomab in induction/consolidation are reported. Among 63 patients with a median follow-up of 53 months, disease-free survival, overall survival, and event-free survival rates are 75.8%, 80.7%, and 74.6%, respectively. A subset of patients continued tyrosine kinase inhibitor therapy without chemotherapy or transplant, with 93.1% achieving molecular response and 28 remaining in long-term complete hematologic response. Allogeneic transplant in first complete hematologic response was performed mainly in patients with persistent minimal residual disease, with 83.3% achieving continuous complete hematologic response.
https://doi.org/10.1200/JCO.23.010
Nelarabine-containing salvage therapy and conditioning regimen in transplants for pediatric T- ALL
In a retrospective analysis of eight pediatric patients with relapsed or refractory T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL), treatment with nelarabine (NEL) plus etoposide, cyclophosphamide, and intrathecal therapy, administered 3 days apart, resulted in favorable outcomes. Five patients achieved complete response and three achieved partial response. All patients underwent hematopoietic stem cell transplantation (HSCT) after two cycles of treatment, except for one who received one cycle. Reduced-intensity conditioning regimens including fludarabine, melphalan, and NEL were effective for patients who previously received HSCT. The addition of NEL to reinduction chemotherapy was well-tolerated and beneficial in achieving remission, with manageable toxicity. Overall survival and event-free survival rates at 2 years were 60.0% and 36.5%, respectively.
https://doi.org/10.1007/s12185-023-03701-z
Single agent subcutaneous blinatumomab for advanced acute lymphoblastic leukemia
In a multi-institutional phase 1b trial, subcutaneous (SC) administration of blinatumomabshowed high efficacy and improved convenience for adults with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL). Patients received either 250 μg once daily (QD) for week 1 and 500 μg three times weekly (TIW) thereafter or 500 μg QD for week 1 and 1000 μg TIW thereafter. The primary endpoint of complete remission/complete remission with partial hematologic recovery (CR/CRh) within two cycles was achieved by 85.7% and 92.3% of patients in the respective dose groups. Most patients achieved measurable residual disease (MRD) negativity, and no grade 4 cytokine release syndrome or neurologic events were reported, indicating both high efficacy and acceptable safety of SC blinatumomab in heavily pretreated adults with R/R B-ALL.
https://doi.org/10.1002/ajh.27227
Utility of allogeneic stem cell transplantation for adult Ph+ALL with complete molecular remission
This study evaluated the effectiveness of allogeneic stem cell transplantation (allo-SCT) for Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) patients achieving complete molecular remission (CMR) within three months of first complete remission (CR1). The 5-year adjusted overall survival (OS) and relapse-free survival (RFS) were significantly higher in the allo-SCT group (73% and 70%) compared to the non-SCT group (50% and 20%). Despite higher non-relapse mortality, allo-SCT was associated with lower relapse rates and superior graft-versus-host disease-free, relapse-free survival (GRFS). The findings indicate allo-SCT in CR1 provides superior survival and reduces relapse rates for Ph+ALL patients with early CMR.
https://doi.org/10.1002/ajh.27237
Olverembatinib in combination with venetoclax and dexamethasone for newly diagnosed Ph+ acute lymphoblastic leukemia
In the era of tyrosine kinase inhibitors (TKIs), the standard treatment for Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) involves TKIs combined with chemotherapy and allogeneic stem cell transplantation (allo-HCT), though relapse remains an issue. A phase 1/2 study was designed to evaluate the chemotherapy-free regimen of olverembatinib, venetoclax, and dexamethasone (OVD) for newly diagnosed Ph+ ALL patients. Among the ten patients recruited, all achieved complete remission or incomplete count recovery and negative minimal residual disease, with 90% achieving complete molecular remission within two cycles. The regimen showed promising efficacy, rapid transfusion independence, and acceptable tolerability, suggesting potential as a frontline treatment for Ph+ ALL.
https://doi.org/10.1002/ajh.27289
Pneumocystis jirovecii pneumonia in paediatric acute lymphoblastic leukaemia
Pneumocystis jirovecii pneumonia (PjP) can be life-threatening, particularly in patients with haematological malignancies like acute lymphoblastic leukaemia (ALL). Prophylaxis has reduced morbidity and mortality, but contemporary data on PjP's incidence and clinical course in homogenous populations like children with ALL are limited. In the multi-international AIEOP-BFM ALL2009 trial, PjP was diagnosed in six children (incidence 1/1000), with five cases linked to insufficient prophylaxis.
https://doi.org/10.1111/bjh.19382
Therapy-related acute lymphoblastic leukaemia in women with antecedent breast cancer
Therapy-related acute lymphoblastic leukemia (tr-ALL) often follows chemotherapy and/or radiation for prior malignancies, especially breast cancer, leading to a female predominance. In a review of 37 women with ALL post-breast cancer treatment, 32% had Philadelphia chromosome positivity (Ph+), 22% had KMT2A alterations, and 46% had other cytogenetic changes. Median overall survival (OS) and relapse-free survival (RFS) were 19.4 and 12.9 months, with Ph+ tr-ALL showing superior outcomes compared to KMT2Ar and other cytogenetic groups. Consolidative allogeneic hematopoietic cell transplantation (alloHCT) improved OS and RFS in KMT2Ar patients, suggesting Ph+ tr-ALL may predict better survival, while KMT2Ar outcomes can be improved by alloHCT.
https://doi.org/10.1111/bjh.19432
Factors affecting risk of methotrexate induced neurotoxicity in childhood acute lymphoblastic leukaemia
Methotrexate (MTX), essential in treating childhood acute lymphoblastic leukaemia (ALL) and lymphomas (LBL), can cause serious neurotoxicity with long-term effects. This study aimed to identify the incidence, risk factors, and outcomes of MTX-induced neurotoxicity in a cohort of 622 children (≤14 years) treated on a modified BFM-95 protocol. MTX-induced neurotoxicity was diagnosed in 6.9% of the patients, with higher rates of high-grade toxicity. Recurrence occurred in 11% of those re-challenged with MTX, and 15% had persistent neurological deficits. Risk factors included older age (>5 years), T-cell phenotype, tumour lysis syndrome during induction, baseline renal problems, and CNS leukaemic involvement.
https://doi.org/10.1111/bjh.19559
Blinatumomab for MRD-Negative Acute Lymphoblastic Leukemia in Adults
In a phase 3 trial, 224 patients aged 30-70 with BCR::ABL1-negative BCP-ALL and MRD-negative remission were randomly assigned to receive either four cycles of blinatumomab plus consolidation chemotherapy or chemotherapy alone. At a median follow-up of 43 months, the blinatumomab group showed significantly improved overall survival (85% vs. 68% at 3 years; HR, 0.41; P=0.002) and relapse-free survival (80% vs. 64% at 3 years; HR, 0.53). A higher incidence of neuropsychiatric events was noted with blinatumomab. Thus, blinatumomab addition improved survival in MRD-negative BCP-ALL patients.
https://doi.org/10.1056/NEJMoa2312948
Upfront allogeneic hematopoietic stem cell transplantation for adult T-cell acute lymphoblastic leukemia/lymphoma in first complete remission
This retrospective study evaluated outcomes of upfront allogeneic hematopoietic stem cell transplantation (allo-HCT) in adult T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) patients in first complete remission (CR1). Among 94 patients, 76 received upfront allo-HCT, and 18 received non-upfront allo-HCT. The 5-year overall survival (OS) was significantly higher in the upfront group (54%) compared to the non-upfront group (19%). Similarly, 5-year progression-free survival was better in the upfront group (50% vs. 20%), with a lower relapse rate (32% vs. 64%). Non-upfront allo-HCT and higher HCT-CI were associated with worse outcomes. The study highlights the importance of upfront allo-HCT in improving survival in T-ALL patients.
https://doi.org/10.1007/s00277-024-05716-w
CD9 shapes glucocorticoid sensitivity in pediatric B-ALL
Resistance to glucocorticoids in pediatric B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is linked to CD9- cells, which show higher resistance to prednisone and dexamethasone. CD9 expression directly influences steroid susceptibility, with CD9- cells demonstrating reduced glucocorticoid responsiveness. The MEK inhibitor trametinib enhances glucocorticoid efficacy against CD9- BCP-ALL cells, providing a potential strategy to overcome glucocorticoid resistance.
https://doi.org/10.3324/haematol.2023.282952
Allogeneic stem cell transplantation is still a highly curative therapy in adults with philadelphia chromosome–positive acute lymphoblastic leukaemia
This retrospective study of 292 Ph+ acute lymphoblastic leukemia (ALL) patients compared outcomes between stem cell transplantation (n=216) and TKI-chemo therapy (n=76). Transplantation resulted in significantly better 4-year disease-free survival (DFS, 68% vs. 24%) and overall survival (OS, 72% vs. 47%). Multivariate analysis indicated that male sex, high WBC count, and low platelet count were linked to poorer outcomes, while transplantation improved DFS and OS across all subgroups. In settings with limited access to newer TKIs or immunotherapies, stem cell transplantation remains vital for Ph+ ALL patients.
https://doi.org/10.1007/s00277-024-05682-3
Genomic Determinants of Outcome in Acute Lymphoblastic Leukemia
This study explored relapse risk in childhood acute lymphoblastic leukemia (ALL), finding that genetic subtype, aneuploidy patterns, and specific genomic alterations significantly affect relapse rates. PAX5-altered ALL had a high relapse risk, while certain chromosome gains/losses in high-hyperdiploid ALL were linked to either favorable or poor outcomes. Genomic changes in genes like INO80, IKZF1, and CREBBP were associated with relapse in specific ALL subtypes. Hence, comprehensive genomic analysis is crucial for optimal risk stratification in ALL.
https://doi.org/10.1200/JCO.23.022
Severe steroid-related neuropsychiatric symptoms during paediatric acute lymphoblastic leukaemia therapy
Steroid-related neuropsychiatric symptoms (SRNS) are a significant concern during acute lymphoblastic leukemia (ALL) treatment, affecting 5.2% of patients in an international database study. Symptoms, commonly psychosis, agitation, and aggression, peaked during induction and intensification phases, with dexamethasone implicated in 86% of cases. Most patients (87%) required pharmacological management, and 38% were hospitalized. Recurrence was reported in 29% patients.
https://doi.org/10.1111/bjh.19610
Obecabtagene Autoleucel in Adults with B-Cell Acute Lymphoblastic Leukemia
Obecabtagene autoleucel (obe-cel), a CAR T-cell therapy targeting CD19, was evaluated in adults with relapsed/refractory B-cell ALL. In the cohort, 77% achieved remission, with a median event-free survival of 11.9 months and overall survival of 15.6 months. Severe cytokine release syndrome occurred in 2.4% and neurotoxicity in 7.1%. Obe-cel showed durable efficacy with low toxicity.
https://doi.org/10.1056/NEJMoa2406526
Efficacy and safety of olverembatinib in adult BCR::ABL1-positive ALL with T315I mutation or relapsed/refractory disease
Olverembatinib, a third-generation TKI, showed promising efficacy in BCR::ABL1-positive ALL, particularly in cases with the T315I mutation. Among relapsed/refractory patients, 71.4% achieved an overall response, while 60.0% of MRD-positive patients attained MRD flow negativity. Median event-free survival was 3.9 months for relapsed/refractory cases and 11.5 months for MRD-positive cases. The manageable safety profile and improved outcomes with stem cell transplantation highlight its potential alongside ponatinib.
https://doi.org/10.1111/bjh.19804
Combination of Ponatinib and Blinatumomab in Philadelphia Chromosome-Positive ALL
A chemotherapy-free regimen combining blinatumomab and ponatinib was evaluated in 60 newly diagnosed Philadelphia chromosome-positive ALL patients. At 24 months median follow-up, the complete molecular response rate was 83%, with measurable residual disease negativity in 98%. Only two patients underwent HSCT. The 3-year overall survival and event-free survival rates were 91% and 77%, respectively. Despite some adverse events, this regimen showed high efficacy and minimized the need for intensive chemotherapy or HSCT in most cases.
https://doi.org/10.1200/JCO.24.002
Inotuzumab Ozogamicin and low-intensity chemotherapy in older patients with newly diagnosed CD22+ Ph Negative B-ALL
A phase II trial (EWALL-INO) evaluated inotuzumab ozogamicin (InO) as a frontline treatment for older patients (≥55 years) with CD22+ Philadelphia-negative BCP-ALL. Among 131 patients (median age 68), 90% achieved complete remission after induction, with 80% achieving deep MRD negativity (<10⁻⁴). One-year overall survival was 73.2%, relapse-free survival was 66%, and relapse incidence was 25%. High-risk cytogenetics and low CD22 expression correlated with worse outcomes. InO showed promising efficacy and safety, supporting its use in this population.
https://doi.org/10.1200/JCO.24.0049
A pediatric-inspired regimen for adolescent and adult patients with Philadelphia chromosome-negative acute lymphoblastic leukemia
A prospective study of 415 Chinese patients aged 14-65 years with Philadelphia chromosome-negative acute lymphoblastic leukemia (Ph– ALL) treated with the pediatric-inspired IH-2014 regimen reported 5-year overall, disease-free, and event-free survival rates of 53.8%, 51.1%, and 45.0%, respectively, after a median follow-up of 40.8 months. The regimen was well tolerated, with a chemotherapy-related mortality rate of 3.6%. Age ≥40 years and persistent minimal residual disease (MRD) after induction were independent prognostic factors. Study shows high-risk patients achieving deep MRD responses post-induction may not benefit from allogeneic stem cell transplantation.
https://doi.org/10.3324/haematol.2023.284228
Inotuzumab ozogamicin combined with chemotherapy in pediatric B-cell precursor CD22+ acute lymphoblastic leukemia
The ITCC-059 trial tested inotuzumab ozogamicin (InO) combined with chemotherapy in relapsed/refractory pediatric CD22+ B-cell precursor acute lymphoblastic leukemia (BCP-ALL). Among 30 patients, the recommended phase II dose (RP2D) was 1.8 mg/m²/cycle (reduced to 1.5 mg/m²/cycle post-remission) with fractioned administration. The pooled response rate was 80%, with 66.7% achieving minimal residual disease negativity. Liver toxicity was observed at higher dexamethasone doses, but reduced dosing improved tolerability. This regimen demonstrated efficacy similar to single-agent cohorts of InO.
https://doi.org/10.3324/haematol.2023.284409
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.