howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Acute Myeloid Leukemia
Updated on: 04.08.2025
Introduction:
- It is a clonal expansion of myeloid blasts in bone marrow, blood and other tissues characterized by presence ≥20% blasts in peripheral smear and/or bone marrow.
- Diagnosis of leukaemia on 20% blast criteria is not itself a therapeutic mandate. Clinical factors must be taken into account when therapeutic decision to treat for AML is made. AML is diagnosed even if blast are <20% when there is associated recurrent cytogenetic abnormalities such as t (8:21) or inv (16).
- For the purpose of diagnosis, abnormal promyelocytes, monoblasts/promonocytes, atypical pronormoblasts and megakaryoblasts are considered as blast equivalents.
Epidemiology:
- Incidence of acute leukaemia: 4 / 1 lac (70% are AML and 30% are ALL)
- Incidence of AML
- 2-3 / 1 lac / year – In children
- 15 / 1 lac / year – In older adults
- Accounts for 1% of all new cancer cases
- Accounts for 20% of acute leukaemia in children and 80% of acute leukaemia in adults.
- Peak in 7th decade
Etiology: Exact etiology is not known
- Environmental factors
- Solvents (benzene)
- Smoking
- Ionizing radiation: Atomic bomb exposure, nuclear power exposure, medical radiation
- Chemotherapy- Alkylating agents, Topoisomerase II inhibitors
- Genetic disorders
- Congenital defects: Down syndrome, Bloom syndrome, Monosomy 7 syndrome, Klinefelter syndrome, Turner syndrome, Neurofibromatosis, Congenital dysmorphic syndromes
- Marrow failure syndromes- Fanconi anemia, Dyskeratosis congenita, Schwachman-Diamond syndrome, Amegakaryocytic thrombocytopenia, Blackfan-Diamond syndrome, Kostmann agranulocytosis
- Pre-existing clonal disorders- MPN, MDS, PNH, Myeloma
Pathogenesis:
- Leukemogenesis is a multistep process
- Class 1 mutations
- Induce proliferation and give survival advantage without affecting differentiation
- Include- FLT3, KIT, RAS, PTPN11, JAK2
- Class 2 mutations
- Responsible for impaired differentiation and subsequent apoptosis
- Include- PML-RARA, RUNX1, MLL, CEBPA, NPM1
- Finally these 3 pathways are involved in blast proliferation
- PI3K-AKT
- RAS-RAF-MEK-ERK
- STAT3
- Class 1 mutations
- Normal hematopoiesis is inhibited by
- Physically replacing the normal marrow precursors
- Cellular / humoral mediated mechanisms
- Mutations seen in cases of AML
- Core binding factor rearrangements:
- Seen in 15% of AML
- Linked with favourable prognosis
- CBF regulates expression of IL3, GM-CSF and M-CSF receptor.
- Translocations involving CBF include inv (16), t (16:16), t (8:21), t (3:21) and t (12:21)
- NPM1 mutation:
- Located on chromosome 5q21
- Encodes a nucleus-cytoplasmic shuttling protein
- This mutation is seen in 1/3rd of AML patients
- Often seen in AML with normal cytogenetics
- If seen alone, has good prognosis. But if associated with FLT3 mutation then carries bad prognosis.
- Over 30 mutations have been described
- Morphologically myelomonocytic/ monocytic leukaemia
- Lack of CD34 is diagnostic of this mutation
- FLT3 mutation:
- Encodes tyrosine kinase receptor
- Internal tandem duplicates (ITD) on chromosome 13 are seen in 1/3rd of AML
- Associated with poor prognosis
- KIT Mutations
- Carry unfavourable prognosis
- TKI may be considered in these cases
- CEBPA Mutations
- Seen in 6-15% of AML
- Usually seen in AML with normal cytogenetics
- Good prognosis
- Morphologically- AML with maturation/ without maturation
- CD7 is positive in 50-70% patients
- Core binding factor rearrangements:
WHO Classification:
- Acute myeloid leukaemia with defining genetic abnormalities (If AML is therapy related, then even if defining genetic abnormality is present, it should be treated as MDS/AML- Therapy related. Presence of additional cytogenetic abnormalities or additional somatic mutations such as NRAS, ASXL1, TET2 etc, does not change diagnosis of AML with particular defining genetic abnormality)
- Acute promyelocytic leukaemia with PML::RARA fusion
- Acute myeloid leukaemia with RUNX1::RUNX1T1 fusion
- Acute myeloid leukaemia with CBFB::MYH11 fusion
- Acute myeloid leukaemia with DEK::NUP214 fusion
- Acute myeloid leukaemia with RBM15::MRTFA fusion
- Acute myeloid leukaemia with BCR::ABL1 fusion
- Acute myeloid leukaemia with KMT2A rearrangement
- Acute myeloid leukaemia with MECOM rearrangement
- Acute myeloid leukaemia with NUP98 rearrangement
- Acute myeloid leukaemia with NPM1 mutation
- Acute myeloid leukaemia with CEBPA mutation (includes biallelic as well as single mutations located in the basic leucine zipper (bZIP) region of the gene)
- Acute myeloid leukaemia, myelodysplasia-related
- Acute myeloid leukaemia, defined by differentiation
- Acute myeloid leukaemia with minimal differentiation
- Acute myeloid leukaemia without maturation
- Acute myeloid leukaemia with maturation
- Acute basophilic leukaemia
- Acute myelomonocytic leukaemia
- Acute monocytic leukaemia
- Acute erythroid leukaemia
- Acute megakaryoblastic leukaemia
- Myeloid sarcoma
Clinical Features:
- Due to marrow suppression
- Anemia
- Leukopenia – Results in repeated infections
- Thrombocytopenia – Leads to petechiae and bleeding
- Due to marrow expansion and infiltration of subperiosteum
- Sternal tenderness
- Bone pain
- Due to organ infiltration
- Lymphadenopathy
- Hepatomegaly
- Splenomegaly
- Gum hypertrophy
- Granulocytic sarcoma / Chloroma – Mass lesion in any soft tissue. It is greenish because of presence of myeloperoxidase. Usually seen in skin, orbit, paranasal sinuses, bone, chest wall, breast, heart, GIT, lymph node and spleen.
- Cranial nerve palsies
- Lytic bone lesions, joint pain
- Systemic symptoms- Fever, malaise, weight loss
- Metabolic derangements:
- Hyperuricemia
- Hyponatremia- Due to SIADH, osmotic diuresis due to urate excretion
- Hypokalaemia- Due to kaliuresis
- Hyperkalemia- Due to tumour lysis
- Hypercalcemia- Due to bone resorption
- Hypocalcemia- Due to tumour lysis
- Acid base imbalance
- Hyperleucocytosis
- Seen in 5% patients
- Seen when counts are >1lac/cmm
- Following are manifestations which occur due to vascular invasion and occlusion
- CNS- Dizziness, stupor
- Lungs- Dyspnea
- Penis- Priapism
- Marrow necrosis- Leads to bone pain, fever, anemia, thrombocytopenia, elevated LDH and alkaline phosphatase
Investigations:
- Hemogram:
- Normocytic normochromic anaemia
- Nucleated erythrocytes may be seen
- Variable anisocytosis and poikilocytosis
- WBCs count is usually increased, but may be normal or decreased
- Minimum 20% of blasts/ blast equivalent cells must be there in PB/ BM for diagnosis of AML
- Morphology of tumour cells
- Myeloblasts
- Size is almost equal to that of monocyte
- Nucleus is around to oval, with delicate nuclear chromatin and 2-4 nucleoli.
- Cytoplasm is abundant and basophilic, contains fine azurophilic, myeloperoxidase positive granules, and distinctive red stained rod like structures known as Auer rods.
- Auer rods are present in more than 50% of AML cases. They are abnormal primary granules and stain positively for MPO and acid phosphatise
- Phi bodies are morphologic variant of Auer rods. They are inclusion bodies found in AML, that stain positive for hydroperoxidase.
- Type I myeloblasts: Typical myeloblasts with lacy open chromatin and prominent nucleoli and immature deep blue cytoplasm without granules
- Type II Myeloblasts- Similar to type I except for presence of up to 20 discrete azurophilic granules
- Type III myeloblasts- Similar to typical myeloblasts but numerous azurophilic granules are present
- Abnormal promyelocytes (See chapter on APML)
- Monoblasts
- Medium to large cells with abundant cytoplasm which is moderately to intensely basophilic.
- May show pseudopod formation
- Scattered fine azurophilic granules and vacuoles may be present
- Nuclei are round with delicate, lacy chromatin. They contain one / more large prominent nucleoli
- Promonocytes
- More irregular and delicately convoluted nucleus with fine lace-like chromatin and inconspicuous nuclei
- Cytoplasm – Less basophilic and sometimes vacuoles
- Occasionally contain azurophilic granules
- Atypical erythroblasts
- All stages of erythroid precursors seen
- Dysplastic erythroid precursors
- Erythroblasts – Medium to large sized cells with round nuclei with fine chromatin and parachromatin arranged in tortoise pattern
- Some may contain more than one nuclei
- Some show megaloblastoid changes
- Cytoplasm is deeply basophilic, often granular and contains poorly demarcated vacuoles which are PAS positive material.
- Myeloblasts are also seen in acute erythroleukemia
- Megakaryoblasts
- Medium to large sized cells – 12 – 18 µ
- Nucleus- Round with slight indentation, fine reticular chromatin and 1 to 3 nucleoli
- Cytoplasm is basophilic, often granular and shows distinct blebs / pseudopod formation
- Sometimes small blasts are seen which resemble lymphoblasts.
- Sometimes extensive marrow fibrosis is present
- Myeloblasts
- Other Peripheral smear findings
- Monocytosis
- Neutropenia
- Dysplastic neutrophils – Pseudo Pelger – Huet anomaly and hypogranulation
- Variable eosinophilia and basophilia
- Thrombocytopenia
- Hypogranular, giant forms of platelets may be present
- Bone marrow aspiration and biopsy:
- Hypercellular
- >20% blasts/ blast equivalents
- Dysplastic features may be present
- Normal haematopoiesis is markedly suppressed
- Cytochemistry
- Myeloperoxidase – positive in AML
- Sudan black B- positive in AML
- Alpha naphthyl acetate esterase – positive in monocytic leukaemias
- PAS-Highlights presence of dysplastic erythroblasts and megakaryocytes
- Immunophenotyping by flow cytometry:
- Positive:
- Myeloid markers- MPO, CD13, CD33
- Precursor markers- HLA DR, CD34
- Monocytic markers- CD11b, CD14
- Erythroid markers- Glycophorin A, CD71
- Megakaryocytic markers- CD41, 42
- Negative- T, B markers
- Positive:
- Serum uric acid and LDH level – Raised
- Blood calcium level – Raised due to bone resorption
- Serum and urine muramidase levels – Raised in case of monocytic leukaemia
- Expression on leukaemia blasts of the multidrug resistance glycoprotein (MDR1)
- Molecular studies for FLT3, NPM1, KIT, CEBPA, WT1 etc.
Criteria for Diagnosis:
- ≥20% blasts in peripheral smear and/or bone marrow/ <20% blasts with recurrent cytogenetic abnormalities (Except AML with BCR:ABL rearrangement and AML with CEBPA mutation) AND
- Presence of Auer rods/ MPO stain positive in >3% of blasts (by flow/actual staining)
Diagnostic algorithm for acute myeloid leukemia
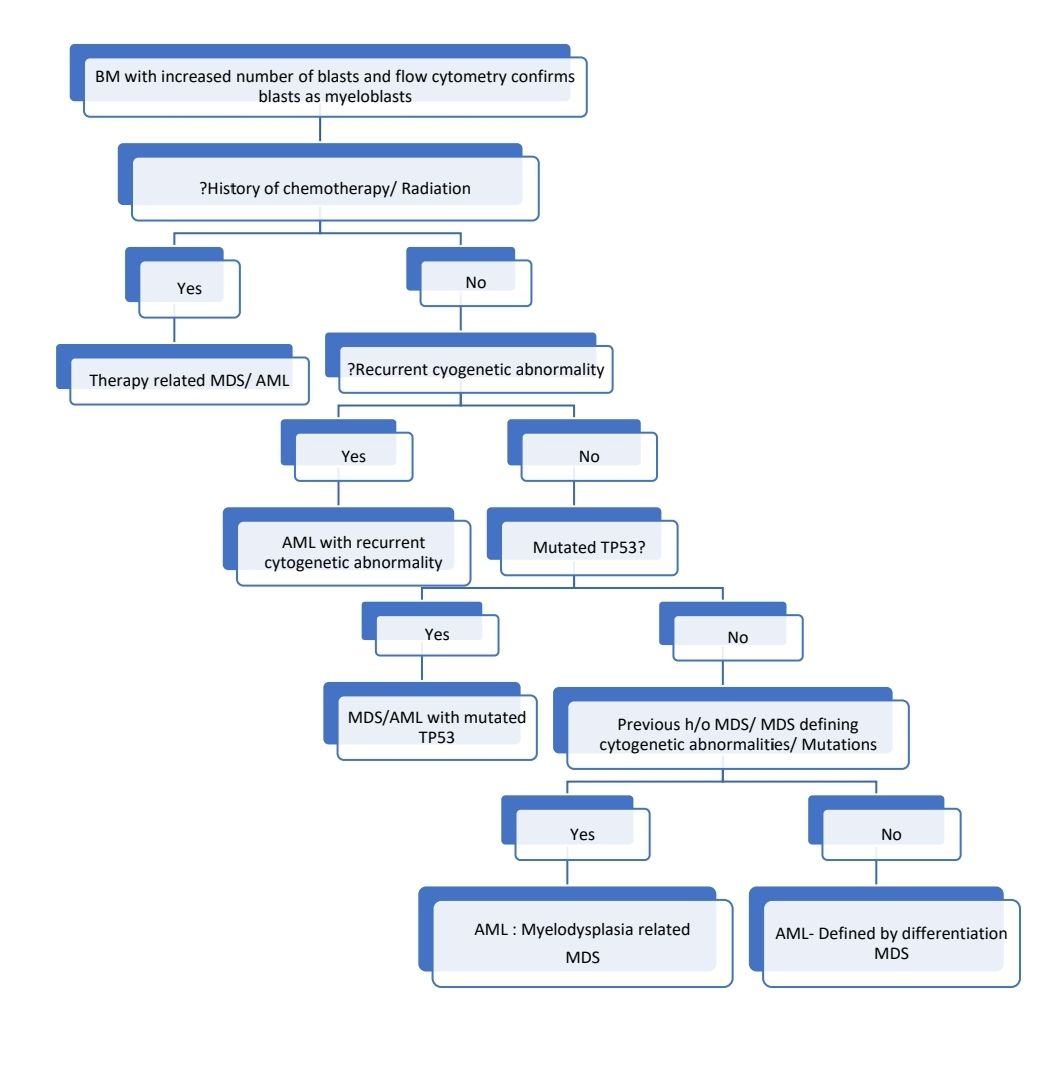
Prognosis:
- Average survival without treatment- 6 weeks
Risk stratification (Applicable to patients who are being treated with intensive protocols with curative intent)
ELN Risk Group | Genetic Abnormality |
Favorable | t(8;21)(q22;q22.1)/RUNX1::RUNX1T1 inv(16)(p13.1q22) t(16;16)(p13.1;q22)/CBFB::MYH11 Mutated NPM1without FLT3-ITD and without adverse cytogenetic abnormalities bZIP in-frame mutated CEBPA (irrespective of whether they occur as monoallelic or biallelic mutations) |
Intermediate | Mutated NPM1 with FLT3-ITD Wild-type NPM1 with FLT3-ITD (without adverse-risk genetic lesions) t(9;11)(p21.3;q23.3)/MLLT3::KMT2A Cytogenetic and/or molecular abnormalities not classified as favorable or adverse |
Adverse | t(6;9)(p23.3;q34.1)/DEK::NUP214 t(v;11q23.3)/KMT2A-rearranged t(9;22)(q34.1;q11.2)/BCR::ABL1 t(8;16)(p11.2;p13.3)/KAT6A::CREBBP inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/GATA2, MECOM(EVI1) t(3q26.2;v)/MECOM(EVI1)-rearranged -5 or del(5q); -7; -17/abn(17p) Complex karyotype (≥ three unrelated chromosome abnormalities in the absence of other class-defining recurring genetic abnormalities; excludes hyperdiploid karyotypes with three or more trisomies (or polysomies) without structural abnormalities) Monosomal karyotype (presence of two or more distinct monosomies (excluding X or Y), or one single autosomal monosomy in combination with at least one structural chromosome abnormality, (excluding core-binding factor AML)) Mutated ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, and/or ZRSR2 (These mutations do not confer adverse risk in patients with favorable risk-defining aberrations) Mutated TP53 (variant allele frequency ≥ 10%) FLT3-ITD- mutated AML with non-availability of FLT3 inhibitors for treatment |
Risk stratification for patients being treated with Venetoclax + HMA
Risk Category | Genetic abormality | Median OS |
Adverse | TP53 mutations | 5.5 months |
Intermediate | No TP53 mutation but harbour FLT3-ITD, NRAS or KRAS mutations | 12.1 months |
| Favourable | Without any of these 4 mutations | 26.5 months |
- Other poor prognostic factors (Factors associated with high risk of relapse)
- Prior myelodysplastic syndrome
- Therapy related AML
- Relapsing AML
- FLT3 ITD Positive
- Mutant TET2, MLL-PTD, DNMT3A, ASXL1, PHF6
- MLL Mutation
- KIT mutation with (8:21)
- WT1 mutation
- High EVI1 expression of mutations
- IDH 1 and IDH2 mutations
- Age >60 years
- Poor performance status
- Associated comorbidities- Obesity, diabetes, chronic renal failure
- Organ dysfunction (hepatic/ renal/ cardiac)
- Failure to achieve CR after induction
- High count at diagnosis (>50,000/cmm)
- Resistance proteins- Ex:P-Glycoprotein
- High LDH
- Need for intubation and ventilatory support during induction
- CD56 expression
- Suboptimal response to induction chemotherapy
Pretreatment Work-up:
- History
- Examination
- WHO P. S.
- BSA
- Haemoglobin
- TLC, DLC
- Platelet count
- BMA and Bx (May be avoided in old patients with high counts)
- AML Morphological Subtype
- Flow cytometry
- Coagulation tests: PT: APPT: Fibrinogen:
- LFT: Bili- T/D SGPT: SGOT:
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid
- LDH
- HIV
- HBsAg
- HCV
- UPT
- Cytogenetics
- PCR for BCR-ABL1
- Multi-target NGS panel including minimum of: IDH1/2, TP53, ASXL1, and RUNX1 (Preferably: NPM1, CEBPA, RUNX1, FLT3, IDH1, IDH2, KIT, WT1, ASXL1, SRSF2, STAG2, RAD21, TP53, KRAS, NRAS, MLL (KMT2A)-PTD, and PPM1D)
- Prognostic category
- ECHO/ MUGA Scan: LVEF- %
- Number of siblings
- HLA typing for HSCT fit patient
- MRI Brain with contrast (in suspected case of leukemic meningitis)
- Whole body PET/CT (If extramedullary disease is suspected)
- LP- CSF with TIT
- If symptomatic
- After 1st consolidation in patients with monocytic differentiation, mixed phenotype acute leukaemia, WBC count >100K at diagnosis, extramedullary disease, FLT3 mutation, positive for CD7 and CD56
- Should be done after coagulopathy is corrected
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
- Consider 1 dose of Cytarabine if hydroxyurea fails to control hyperleukocytosis (Prior to all diagnostic test results are available)
- Day 14 BM study
- Remission BM study
Treatment Plan:
- Principle of therapy for fit individuals: Induction of CR by intensive chemotherapy followed by consolidation using chemotherapy and/or alloSCT
- Applies to patients with myeloid sarcoma as well. Local RT may be used for residual disease in these patients)
- FLT3 mutated AML, AML- Myelodysplasia related and therapy related AML have been discussed separately.
- Fitness for intensive therapy is based on:
- Age
- Comorbidities (Charlson comorbidity index/ Haematopoietic Cell Transplantation–specific Comorbidity Index)
- Cognitive functions
- Level of physical activity (Performance score)
- Psychological health
- Nutritional status
- Social support
- Wish of patient
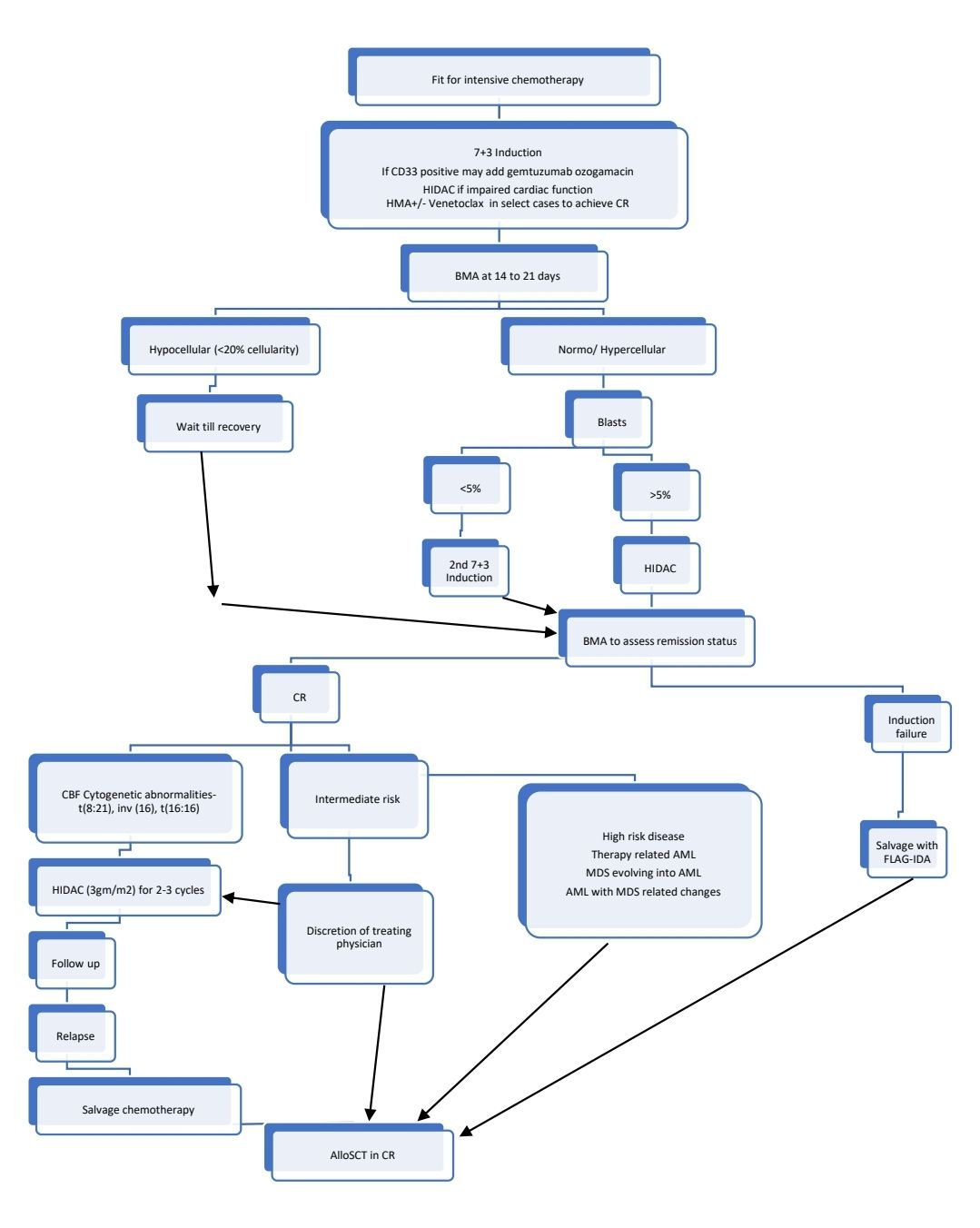
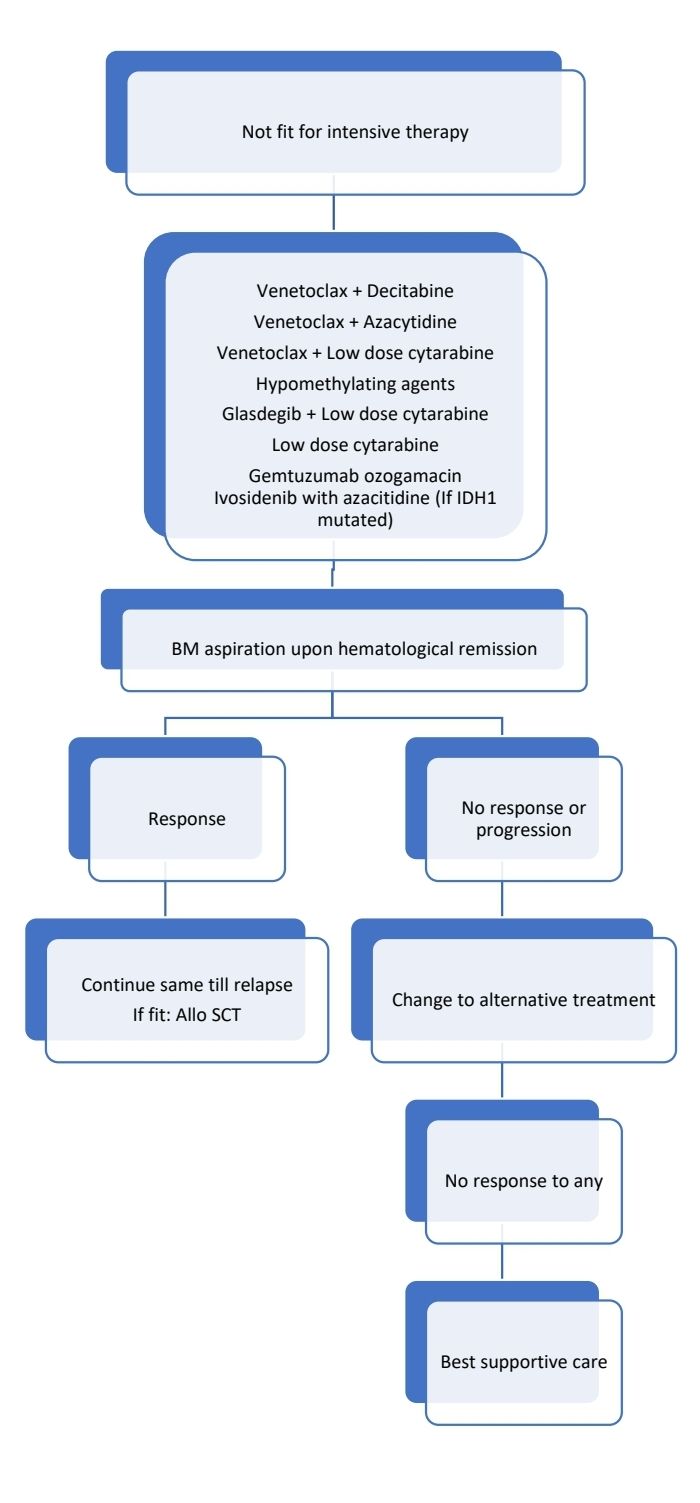
Response Criteria:
- Complete remission
- Means approximate 2-log reduction in tumour burden
- Bone marrow appears normal both morphologically and functionally and is able to produce normal numbers of circulating cells
- Criteria to call as CR
- <5% blasts in an aspirate with spicules
- No blasts with Auer rods
- Peripheral neutrophil count of > 1.5 x 109/L & PL count of > 100 x 109/Lit
- Absence of extramedullary disease
- If there is any doubt about residual leukaemia, BM should be repeated after 1 week.
- Patients should be off G-CSF for a minimum of 7 days before obtaining BM to document remission
- Relapse: Defined as reappearance of
- Leukemic cells in peripheral blood or finding of >5% blasts in bone marrow, not attributable to any other cause. Ex: BM regeneration after consolidation therapy etc.
- Extramedullary disease
- Minimal residual disease (MRD) in treatment of AML: 2 methods (Both can detect one malignant cell in 104 cells). The diagnostic sample should ideally be obtained from the bone marrow aspirate.
- Flow cytometry:
- Aberrant combinations of surface antigens are used
- Immunophenotyping may change at relapse
- No clear cut-off to call as MRD- Positive. Generally>0.05% is indicative of high risk of relapse.
- RT- qPCR for abnormal fusion genes
- Mostly used in patients with CBF or NPM1 mutations
- Other techniques:
- Next generation sequencing (NGS)
- Digital droplet PCR
- MRD measurement is useful both in patients treated with intensive and less-intensive chemotherapy. MRD positive patients have high risk of relapse and worse overall survival.
- There is lack of data precludes firm therapeutic recommendation based on the MRD result at the end of treatment. If MRD is positive at the end of 2 cycles of intensive chemotherapy, some experts consolidate such patients with alloSCT.
- Flow cytometry:
About Each Modality of Treatment:
- 7+3 Induction
- Inj. Cytarabine 100 (preferred)-200 mg/m2 in 500ml NS over 24 hrs from day 1 to day 7.
- Inj. Daunorubicin 60 (preferred) -90 mg/m2 in100ml NS over 1hr from day 1 to day 3.
- Idarubicin may be given instead of Daunorubicin. Dose:12 mg/m2 for 3 days.
- Mitoxantrone may be given instead of Daunorubicin. Dose:12 mg/m2 for 3 days
- Start G-CSF after day 14 BMA
- Give Posaconazole/ Amphotericin prophylaxis
- Dose adjustments: None
- 50-85% of patients achieve remission
- CPX- 351, a liposomal formulation of Cytosine and daunorubicin is found to be better in t-AML and AML with MDS related changes.
- Gemtuzumab ozogamicin (CD33 monoclonal antibody conjugated to the toxin calicheamicin) 3 mg/m2 (up to one 4.5-mg vial)- single dose On day 4. No overall survival advantage except in patients with favourable risk cytogenetics, hence not used routinely. May cause hepatotoxicity, hence not routinely added to induction chemotherapy.
- Other induction chemotherapy regimens include:
- CLAG-M: Cladribine 5 mg/m2 IV d1–5; cytarabine 2000 mg/m2 IV d1–5 (starting 2h after cladribine infusion); mitoxantrone 10 mg/m2 IV d1–3; G-CSF 300 mg SC d0–5
- FLAG-IDA- See below
- MEC: Mitoxantrone 8 mg/m2 IV d1-5; etoposide 100 mg/m2 IV d1-5; cytarabine 1000 mg/m2 IV d1-5
- Venetoclax combined with cladribine, idarubicin, and cytarabine
- Hypomethylating agents:
- Decitabine
- Inj. Decitabine- 20mg/m2- in 300ml NS over 3 hrs- From Day 1 to Day 5
- Frequency: 28 days
- Dose adjustments:
- S. Creatinine- >2mg/dL- Hold until recovery of toxicity
- Bilirubin- >2gm/dL- Hold until recovery
- ANC <1000/cmm or platelet count <50,000/cmm- Hold until CBC values increase to more than cut off values (this applies only if, baseline ANC was >1500/cmm and baseline platelet count was >75,000/cmm)
- Active infection- Hold until recovery
- Azacytidine
- Inj. Azacytidine- 75mg/m2- SC- OD- Divide doses if volume is >4ml.- From Day 1 to Day 7
- Frequency: 28 days
- Dose adjustments:
- S. Creatinine- >3mg/dL- Hold until recovery to normal and then restart with 50% of dose.
- Hepatic impairment- Use with caution
- ANC <1000/cmm or platelet count <50,000/cmm- Hold untill CBC values increase to more than cut off values and then give 50% of dose. (this applies only if, baseline ANC was >1500/cmm and baseline platelet count was >75,000/cmm)
- Active infection- Hold until recovery
- Decitabine
- Low dose cytarabine
- Frequency- 28 to 42 days (Given for minimum for 4 cycles. If appropriate continued indefinitely)
- Inj. Cytarabine- 20mg- SC- BD- for 10 days
- Dose adjustments:
- Bilirubin/ SGPT/SGOT- >2x ULN- Use with caution
- Venetoclax:
- Used along with hypomethylating agents especially Azacitidine (VIALE-A Trial)
- Inhibits BCL2 (pro-apoptotic protein) and induces apoptosis in malignant cells
- Especially useful in AML with TP53 mutations
- Used even in fit patients, as CR rates are high and aceeptable toxicity allows early allo-SCT leading to better long-term outcomes.
- Given PO- once daily
- Maximum number of cycles is not known
- To decrease the risk of TLS, decrease the WBC count to <25 x 109/L with hydroxyurea prior to starting venetoclax.
- 100mg d1, 200mg d2, 400mg d3 and beyond
- Generally given for 7-14 days in a 28 days cycle to allow haematological recovery.
- If used with posaconazole or other CYP3A4 inhibitor, reduce dose from 400mg to 70mg.
- For mild to moderate hepatic/ renal impairment, no dose adjustments are necessary. Hold if grade 3-4 dysfunction.
- For 1st cycle, Continue treatment regardless of cytopenias; transfuse as needed and no growth factors until treatment cycle is complete.
- BM biopsy for response assessment on days 21–28:
- If no morphologic remission (persistent BM blasts above 5%) but evidence of efficacy exists, proceed with a second cycle without interruption with the goal of achieving morphologic remission, and repeat BM biopsy on days 21–28 of this cycle. If no response after 2-3 cycles, stop Venetoclax and consider alternate therapy/ clinical trial.
- If blasts are <5%: Start growth factors as needed and wait till recovery. Resume next cycle after count recovery (ANC >1,000/cmm and Platelet count >75,000/cmm). Repeat BM if no count recovery by Day 42.
- Cytopenia is the most common side effect. Need to decrease the dose as per following protocol.
- Dose at interruption 400 mg: Restart at 300 mg
- Dose at interruption 300 mg: Restart at 200 mg
- Dose at interruption 200 mg: Restart at 100 mg
- Dose at interruption 100 mg: Restart at 50 mg
- Dose at interruption 50 mg: Restart at 20 mg
- Dose at interruption 20 mg: Restart at 10 mg
- Where there are delays in count recovery, reduction in duration of venetoclax and/or reduction in dose or duration of HMA or LDAC should be considered.
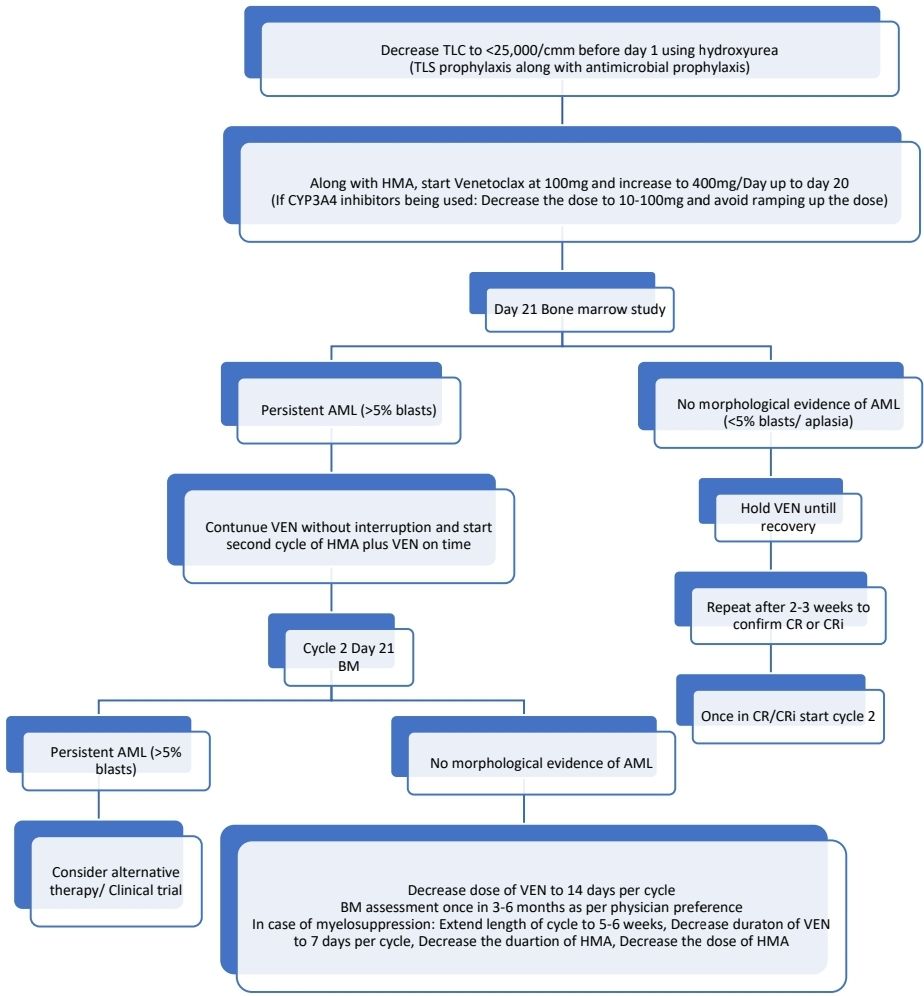
- FLAG-IDA
- Inj. Fludarabine- 30mg/m2 in 100ml NS over 30min- from day 1 to day 5.
- Inj. Cytarabine 2000mg/m2 in 500ml NS over 4hrs. Start 4 hours after completion of fludarabine. From day 1 to day 5.
- Inj. Idarubicin- 8mg/m2- slow IV bolus in running saline- From day 3 to day 5.
- Inj. G-CSF- 300mcg- SC- BD from day 1
- Dose adjustments:
|
| Fludarabine | Cytarabine | Idarubicin |
Creatinine clearance (ml/min) | 45-70 | Give 50% of dose | Give 60% of dose |
|
| 30-45 | Give 50% of dose | Give 50% of dose |
|
| <30 | Do not give | Do not give |
|
Creatinine (mg/dL) | 1.3- 1.97 |
|
| Give 50% of dose |
| >1.97 |
|
| Clinical decision |
Bilirubin (mg/dL) | 2-5 | --- | Give 50% of dose | Give 50% of dose |
| >5 | --- | Clinical decision | Clinical decision |
- Other salvage protocols:
- Cladribine 5 mg/m2 on days 2–6; cytarabine 2 g/m2 over 4 hours starting 2 hours after cladribine infusion on days 2–6; G-CSF SC daily on days 1–6 +/- (mitoxantrone 10 mg/m2 or idarubicin 8 mg/m2 on days 2–4)
- Cytarabine 2 g/m2 every 12 hours on days 1–6 or 3 g/m2 every 12 hours x 4 days vs. days 1, 3, 5, 7 with (daunorubicin 50 mg/m2 or idarubicin 12 mg/m2 x 3 days)
- Cytarabine 1.5–3 g/m2 every 12 hours on days 1–6 +/- mitoxantrone 10 mg/m2 on days 7–9
- Etoposide 100 mg/m2 on days 1–5; cytarabine 3 g/m2 every 12 hours on days 1–4
- Etoposide 100 mg/m2 on days 1–5; cytarabine 1 g/m2 every 12 hours on days 1–5; mitoxantrone 8 mg/m2 daily on days 1–5
- Clofarabine 40 mg/m2 on days 1–5
- Clofarabine 25 mg/m2 on days 1–5; cytarabine 2 g/m2 over 3 hours starting 4 hours after clofarabine infusion on days 1–5
- Cladribine 5 mg/m2 on days 1–5; cytarabine 1–1.5 g/m2 over 2 hours starting 3–6 hours after cladribine infusion on days 1–5; idarubicin 10 mg/m2 on days 1–3; venetoclax 400 mg on days 2–8
- High dose Cytarabine (HIDAC)
- Frequency: 28 days
- Inj. Cytarabine 1,500-3000mg/m2 in 500ml NS over 4 hours- BD on Days 1, 3, 5
- Prednisolone eye drops- QID
- Look for cerebellar toxicity including tests for nystagmus, slurred speech, and dysmetria. In patients who develop cerebellar toxicity, cytarabine should be stopped. Rechallenge with HiDAC in future treatment cycles should not be attempted
- Dose adjustments:
- Creatinine clearance (ml/min)
- 45-60- Give 60% of dose
- 30-45- Give 50% of dose
- <30- Do not give
- Bilirubin- >2gm/dl- Give 50% of dose
- Creatinine clearance (ml/min)
- IDH 1 Inhibitors (Used as monotherapy or along with Azacitidine+/- Venetoclax cases with IDH1 mutations. May be used upfront or in relapsed/ refractory situations. Cause differentiation syndrome, which needs to be treated with corticosteroids)
- Ivosidenib- 500 mg daily
- Olutasidenib- 150 mg PO twice daily on days 1–28 of a 28-day cycle
- IDH 2 Inhibitors (Causes differentiation syndrome, which needs to be treated with corticosteroids)
- Enasidenib: 100 mg PO once daily on days 1–28 of a 28-day cycle
- Oral DNMT inhibitor
- CC486- Mostly used as maintenance after intensive chemotherapy
- Hedgehog pathway inhibitor
- Glasdegib- Orally 100 mg/day (Given along with LDAC or induction chemotherapy)
- Menin inhibitor (Useful in R/R KMT2A rearranged acute leukemia i.e. those with 11q23 translocations)
- Revumenib: 160 mg PO twice daily on days 1-28 of a 28-day cycle (with a strong cytochrome P450 inhibitor) or 270 mg PO twice daily on days 1-28 of a 28-day cycle (without a strong cytochrome P450 inhibitor)
Consolidation of CR
- Even in CR there are 109 leukaemia cells in body. Consolidation is needed to reduce this number and minimize chances of relapse.
- Consolidation must be started a week after documentation of remission following induction chemotherapy
- Options of consolidation include:
- 2-4 cycles of HIDAC
- Allo SCT
Allogeneic SCT from HLA compatible sibling donor
- Most effective way to prevent leukaemia relapse (Relapse risk reduced from 45% to 20% due to Graft Vs Leukaemia effect)
- Overall expectation of cure with MSD - 60% and with MUD- 45% (As there are non leukemic causes for death)
- Conditioning regimens used commonly are
- Busulfan with cyclophosphamide
- Treosulfan with fludarabine
- There is no strict age bar. Transplant can be done up to 60-65 years of age, if patient is fit. Reduced intensity protocols are used in them.
- If transplant is being delayed for any reason CR can be maintained by giving intermediate/ high dose cytarabine. (Bridging therapy)
- Indications
- Intermediate and poor risk AML
- FLT3 Positivity
- Relapsed AML
- Compared to chemotherapy alone survivors, BMT survivors have morbidities like GVHD
- Favourable outcome factors
- Male donor
- CMV – negative donor when host is CMV negative
- Higher cell count in graft
- With Haplo transplants cure rates are 45-50%
- Treatment of relapse after allo SCT
- Donor lymphocyte infusions
- 2nd Allo SCT after salvage chemotherapy
- Immune checkpoint inhibitors: Nivolumab, Pembrolizumab etc
- Consider using newer molecules such as IDH inhibitors, Revuminib, Gilteritinib etc
- Gemtuzumab Ozogamycin as monotherapy- 3 mg/m2 on days 1, 4, and 7
- Palliate using HMA/ LDAC +/- Venetoclax
- Autologous SCT- Lacks GVL effect, so not useful in AML
Maintenance therapy:
- No overall improvement in survival has been observed.
- Oral azacytidine has shown some improvement in patients receiving chemotherapy alone treatment. Dose: 300 mg PO daily on days 1–14 of each 28-day cycle (Based on the results of the phase III QUAZAR AML-001 trial)
- Sorafenib (400 mg PO twice daily on days 1–28 ) is useful patients with FLT3-ITD mutations (lowers risk of relapse)
Treatments under clinical trial:
- Magrolimab: Anti-CD47 monoclonal antibody
- Sabatolimab: Anti-TIM-3 antibody (immune checkpoint inhibitor)
- Other menin inhibitors: ziftomenib, bleximenib, BMF-219, DS1594 and DSP 5336
- CART cells directed against CD123, CD33, and CD70
- Agents directed toward CD123: Tagraxofusp and Pivekimab sunirine
- Flotetuzumab: Binds to CD3ε on T cells and CD123
Supportive Care:
- Tumour lysis prophylaxis and monitoring
- CBC daily: SOS- PRBC and platelet transfusions
- Prophylactic oral antibiotics and antifungals (Posaconazole during remission induction therapy)
- Management of febrile neutropenia
- To prevent hyperleucocytosis related complications: If TLC is >1lac/cmm, attempt immediate cytoreduction using Hydroxyurea- 1.5-2.5gm- QID-PO for approximately 36 hours
- When on High dose cytarabine:
- Watch for cerebellar toxicity- If this is seen, stop cytarabine permanently
- Steroid eye drops to prevent chemical conjunctivitis
- Start G-CSF after day 14 BM study. Patient has to be off G-CSF for at least 7 days before obtaining bone marrow to document remission.
Monitoring After Treatment/ Follow-up:
- CBC every 1-3 monthly for 2 years, then every 3-6 monthly for 5 years
- Bone marrow only if PS shows abnormal cells or cytopenias develop
- Late effects of chemotherapy:
- Cardiomyopathy, arrythmias
- Growth failure
- Neurocognitive abnormalities
- Endocrine deficiencies
- Second malignancies
- Decreased fertility
Special Situations:
- Treatment for meningeal leukemia (CNS Leukemia- Mass noted on imaging/ blasts in CSF):
- High dose cytarabine crosses blood brain barrier
- Intrathecal chemotherapy- Triple IT- Should be given concurrently with induction chemotherapy
- Twice weekly, till CSF becomes clear
- Then weekly for 2 months
- Then every 14 days for 2 months
- Then monthly for 1 year
- Cranial irradiation
- Pregnancy with AML
- Incidence- 1 in 75,000 to 1,00,000 pregnancies
- Chemotherapy drugs have maximum teratogenic effect in first trimester; hence chemotherapy has to be given after MTP.
- Intense chemotherapy can be given in 2nd and 3rd trimester, but it can result in
- Premature delivery
- High perinatal mortality
- Low birth weight
- Treatment should never be delayed, as it has adverse effect on both mother and fetus.
- Treatment is same as other AML patients.
- Chemotherapy should be dosed according to actual body weight.
- Close consultation with obstetric team and fortnightly ultrasound scans are necessary to know fetal growth and well-being.
- Use vaginal delivery whenever possible. Planned delivery is always better than spontaneous labor. This should be planned when blood counts are almost normal (usually 3 weeks post chemotherapy).
- If severe neutropenia/ thrombocytopenia, avoid regional block and epidural analgesia.
- Active management of 3rd stage of labor is necessary to decrease the risk of postpartum hemorrhage.
- Remission rates are same as compared to other patients.
- Breast feeding is not recommended when mother is undergoing chemotherapy.
- AML in children
- Protocols with cytarabine, anthracycline and 6-TG are used in induction. Multidrug consolidation with additional agents, such as etoposide, are used in consolidation.
- Remissions are seen in 80% of patients
- 5 year relapse free remission is seen in 50% of children
- AML in less than 1 year of age is treated with interfant-99 protocol.
- Unfavorable factors include:
- Monocytic leukemia
- TLC >1lac/cmm at diagnosis
- FLT3 mutation
- Age less than 2 years or more than 10 years
- Abnormalities of chromosomes 3, 5 or 7.
- Complex karyotypes
- More than 15% blasts on day 14
- Pediatric AML- BFM 2004 Protocol
- Standard risk- t (8:21) or inv (16) with <5% blasts in BM on day 15 without FLT3 ITD mutation- Chemotherapy alone. (AIE induction- AI- haM- HAE- RT- Maintenance)
- High risk-All others- Consider Allo SCT in CR1 if matched sibling is available, otherwise give second induction with HAM and then continue with same protocol (AIE induction- HAM- AI- haM- HAE- RT- Maintenance)
- Induction (AIE):
- Inj. Cytosine- 100mg/m2/ day- Continuous infusion on day 1 and 2
- Inj. Cytarabine- 100mg/m2- in 100ml NS over 30min- BD- From day 3 to day 8.
- Inj. Idarubicin- 12mg/m2/day in in 100ml NS over 4 hrs- on days- 3, 5, and 7
- Inj. Etoposide- 150mg/m2 in 100ml NS over 1 hr- OD- From day 6 to day 8. (Give 6 hrs before cytosine)
- Induction 2- HAM Block (Not given for standard risk patients)
- Inj. Cytosine- 3gm/m2 in 250ml NS over 3 hrs- BD- From day 1 to day 3 (Total 6 doses)
- Inj. Mitoxantrone- 10mg/m2 in 100ml NS over 30min- From day 4 to day 5
- Steroid eye drops
- Consolidation- AI (Start if GC is good, ANC- >1000/cmm, Platelet count >80,000/cmm)
- Inj. Cytosine- 125mg/m2/day- Continuous infusion- From day 1 to day 4
- Inj. Idarubicin- 7mg/m2- in 100ml NS over 1hr- on days 3 and 5.
- Intrathecal cytosine- 40mg- on day 1 and day 6
- Consolidation- haM: (Start if GC is good, ANC- >1000/cmm, Platelet count >80,000/cmm)
- Inj. Cytosine- 1gm/m2 in 250ml NS over 4 hrs- BD- from day 1 to day 3 (Total 6 doses)
- Inj. Mitoxantrone- 10mg/m2- in 100ml NS over 30min- OD- From day 3 to day 4
- Intrathecal cytosine- 40mg- on day 1
- Steroid eye drops
- Intensification- HAE (Start if GC is good, ANC- >1000/cmm, Platelet count >80,000/cmm)
- Inj. Cytosine- 3gm/m2 in 250ml NS- over 4 hrs- BD- From Day 1 to day3 (Total 6 doses)
- Inj. Etoposide- 125mg/m2 in 100ml NS over 1hr- OD from Day 2 to day 5 (Give 6 hrs before cytosine)
- IT- Cytosine- 40mg- on day 1
- Steroid eye drops
- Cranial irradiation- 18G (For children aged >3 years)
- Maintenance – for 12 months
- 6-Mercaptopurine- 40mg/m2- PO- OD- Daily (Adjust the dose to keep WBC count between 2000-3000/cmm)
- Inj. Cytosine- 40mg/m2- SC- OD- Days 1-4 of every month
- Intrathecal cytosine- 40mg- Once in 3 months
- Congenital leukemia
- AML which is seen at less than 4 weeks of age
- Usually have leukemia cutis (Blue berry muffin)
- Skin lesions precede BM manifestations
- Characterized by
- Leucocytosis with blood and marrow blast cells
- Hepatosplenomegaly
- Thrombocytopenia with purpura
- Anemia
- Morphologically, usually monocytic leukemias
- Rarely spontaneous remissions are seen especially in infants with t (8:16)
- Most of the patients do not survive for more than few weeks/ months.
- Treatment is largely ineffective.
- Except for those with t(8:16), for rest chemotherapy has to be started immediately
- HSCT has to be done in CR1
Subtypes of AML
Acute myeloid leukaemia with RUNX1::RUNX1T1 fusion
- Associated with t(8;21)
- Core binding factor is a transcription factor complex comprised of
- DNA-binding CBFα subunit (RUNX1, RUNX2, or RUNX3)
- Non-DNA-binding heterodimerization partner CBFβ subunit (encoded by the CBFB gene).
- RUNX1 (formerly AML1) is a master transcriptional regulator of adult hematopoiesis expressed by all haematopoietic lineages.
- RUNX1::RUNX1T1 fusion results in blocking of myeloid differentiation. Additional mutations such as mutations in DHX15 and ZBTB7A result in development of AML.
- Account for 5-12% of all AML
- Seen in younger patients
- Morphology: Similar to AML M2. Auer rods are frequently found.
- Eosinophilic precursors are increased in BM
- Usually positive for lymphoid marker- CD 19, CD79a and PAX5. CD56 is often positive
- Carries good prognosis when treated with high dose cytarabine.
Acute myeloid leukaemia with CBFB::MYH11 fusion
- Associated with inv (16) or t(16;16)
- MYH11, which codes for a smooth muscle myosin heavy chain, combines with CBFßà CBFB::MYH11 protein interferes with the function of RUNX1/CBFB transcription factors
- Morphologically similar to acute myelomonocytic leukemia with eosinophilia
- Granules of eosinophils are larger than those normally present in immature eosinophils
- Co-expression of CD2 is often present
- Secondary chromosomal aberrations are present in ~45% cases
- Relapse may present with extramedullary disease
- Associated with good prognosis when treated with high dose cytarabine.
Acute myeloid leukaemia with DEK::NUP214 fusion
- Associated with t(6;9)(p22.3;q34.1)
- Mean age- 13 years
- Morphologically AML with maturation/ Acute myelomonocytic leukemia
- Associated with basophilia, pancytopenia and dysplasia
- Poor prognosis
- BMT should be done as a part of treatment
Acute myeloid leukaemia with RBM15::MRTFA fusion
- Also called acute megakaryocytic leukemia with t(1:22)
- Occurs in infants (<3 years)
- Can present as mass
- Morphology and immunophenotyping similar to acute megakaryocytic leukemia
Acute myeloid leukaemia with BCR::ABL1 fusion
- De novo AML with BCR::ABL1 detected at initial diagnosis and where no subsequent evidence of chronic myeloid leukaemia can be established.
- Compared to CML-BP, patients with AML with BCR::ABL1 have higher percentage of blasts, lower percentage of basophils and lower frequency of splenomegaly.
- 50-60% cases demonstrate additional chromosomal alterations
- Morphologically they are AML with minimal differentiation, without maturation, or with monocytic differentiation.
- Poor prognosis. Survival following allogeneic transplantation is similar to intermediate-risk AML
Acute myeloid leukaemia with KMT2A rearrangement
- Earlier called AML with 11q23 abnormalities (MLL gene abnormalities)
- 5 – 6% of AML
- t (9:11) is most common abnormality which is associated with fusion of MLL-T3 with MLL gene.
- Increased frequency in
- Infants with AML
- Therapy related AML (DNA topoisomerase II inhibitors)
- Usually acute monocytic/ myelomonocytic leukemia
- Extramedullary involvement is seen in 1/3rd of patients.
- Associated with poor prognosis
Acute myeloid leukaemia with MECOM rearrangement
- Associated with inv (3) or t(3:3) which results in GATA2::MECOM rearrangement
- May be associated with dysplasia
- Aggressive disease with short survival time
- Peripheral smear
- Thrombocytosis is seen in 1/3rd of patients
- Hypogranular neutrophils with pseudo Pelger-Huet anomaly
- Blasts may be seen in some patients
- Bone marrow
- Increased number of atypical megakaryocytes
- Morphologically non-M3 AML
- Dysplasia seen in all three lineages
- Increased number of eosinophils, basophils and mast cells are noted.
- May respond to Arsenic trioxide and thalidomide
Acute myeloid leukaemia with NUP98 rearrangement
- Involves translocation of chromosome 11p
- More than 40 fusion partners
- Poor prognosis
- Allogeneic stem cell transplantation in CR1 is the treatment of choice
Acute myeloid leukaemia with NPM1 mutation
- Nearly one-third of AML adult patients have NPM1 mutation
- Commonly there is 4 base pair insertion in exon 12 of the gene
- Myelomonocytic or monocytic differentiation is common
- Blasts have cup-like nuclear morphology
- Good response to induction therapy
- Prognosis is influenced by co-occurring mutations
Acute myeloid leukaemia with CEBPA mutation
- By definition, it should be
- Biallelic or,
- If single, must be located in the basic leucine zipper region
- 5% of AML in children and 5-11% in adults.
- Morphologically AML with or without maturation
- Favourable prognosis
Acute myeloid leukemia with FLT-3 mutation
- FLT3is located on chromosome 13q12
- Encodes tyrosine kinase receptor that is involved in hematopoietic cell differentiation
- Seen in 20% cases of AML and MDS
- 2 types of mutations are found
- FLT3 ITD (Internal tandem duplication)
- FLT3 TKD (Tyrosine kinase domain)
- Usually has adverse outcome
- Treatment plan:
- Frail: Options include
- Triplet combination of azacitidine, venetoclax, and gilteritinib
- Azacitidine with venetoclax
- HMA alone
- Frail: Options include
Midostaurin
- FLT3 receptor signalling inhibitor. Also inhibits KIT signalling
- Dose: 50mg- PO- BD on days 8-21 of each cycle of induction (7+3) and consolidation (HIDAC). To be taken with food.
- Side effects: Headache, nausea, interstitial pneumonitis
- Do not use with CYP3A inhibitors/ inducers
- Other FLT3 inhibitors are:
- Quizartinib: 26.5 or 53 mg PO daily on days 1–28 of each 28-day cycle
- Glilteritinib: 80-120 mg/day
- Crenolanib
Acute myeloid leukaemia with other defined genetic alterations
- Includes:
- AML with RUNX1T3(CBFA2T3)::GLIS2
- AML with KAT6A::CREBBP
- AML with FUS::ERG
- AML with MNX1::ETV6
- AML with NPM1::MLF1
AML- Myelodysplasia related (AML-MR)
- It is acute leukemia with > 20% myeloblasts in blood / marrow along with any one of following
- Previous history of MDS or MDS/MPN
- Defining clonal cytogenetic abnormalities:
- Complex karyotype (≥ 3 abnormalities)*
- 5q deletion or loss of 5q due to unbalanced translocation
- Monosomy 7, 7q deletion, or loss of 7q due to unbalanced translocation
- 11q deletion
- 12p deletion or loss of 12p due to unbalanced translocation
- Monosomy 13 or 13q deletion
- 17p deletion or loss of 17p due to unbalanced translocation
- Isochromosome 17q
- idic(X)(q13)
- Defining somatic mutations:
- ASXL1
- BCOR
- EZH2
- SF3B1
- SRSF2
- STAG2
- U2AF1
- ZRSR2
- AML-MR should not be diagnosed solely on the basis of multilineage dysplasia. However, most of these patients do have dysplastic changes in bone marrow.
- Usually seen in elderly patient
- Usually present with pancytopenia
- Prognosis: Poor
- Treatment:
- Induction with CPX-351 (Liposomal cytarabine/ daunorubicin) / 7+3 induction, followed by Allogeneic HSCT
- HMA/Low dose cytarabine with Venetoclax and if fit for transplant Allo HSCT in CR1. If not fit, continue same till relapse.
Acute myeloid leukaemia, defined by differentiation
Acute myeloid leukaemia with minimal differentiation (FAB-AML-M0)
- It is an acute leukemia with no evidence of myeloid differentiation by light microscopy, cytochemistry and flow cytometry (MPO stain- <3% blasts are MPO are positive)
- Flow cytometry-
- Positive for at least two myeloid-associated markers, such as CD13, CD33, and CD117.
- Myeloperoxidase is negative
- Most cases are positive for CD34, CD38, CD117, CD123, and HLA-DR.
- Accounts for 5% of total AML
- Prognosis: Poor
AML- Without maturation (FAB-AML-M1)
- It is a leukemia characterized by high percentage of bone marrow blasts with evidence of maturation to, more mature forms. However, mature forms (promyelocytes, myelocytes and neutrophils) comprise of less than 10% of all cells in bone marrow.
- Comprise 10% of total AML
- Median age – 46 years
- Some blasts have azurophilic granules and / or unequivocal Auer rods
- MPO & SBB positivity present in >3% of blasts
- Flow cytometry: Positive for cytoplasmic myeloperoxidase and two or more myeloid-associated antigens such as CD13, CD33, and CD117. Often positive for CD34 and HLA-DR.
- Prognosis: Poor when associated with +8, t (9; 22), t (6; 9), del (5q), monosomy 5
AML-With maturation (FAB-AML-M2)
- It is a leukemia characterized by presence of >20% blasts in blood / marrow, and evidence of maturation to more mature neutrophils (> 10% promyelocytes, myelocytes and mature neutrophils) ; monocytes comprise <20% of bone marrow cells
- Flow cytometry: Positive for ≥2 myeloid-associated markers (e.g. myeloperoxidase, CD13, CD33, CD117).
- Account for 30-45% of AML
Acute basophilic leukaemia
- It is an AML in which primary differentiation is towards basophils
- Some represent blast transformation of CML
- Cutaneous involvement, hepatosplenomegaly, lytic bone lesions, and symptoms related to hyper-histaminaemia may be present
- Blasts/ Immature basophils comprise 20-80% cells in BM:
- Medium to large-sized and characterized by a high N:C ratio
- Nucleus: oval, round, or irregular with dispersed chromatin, and one to several prominent nucleoli.
- Cytoplasm: Coarse basophilic granules.
- Special stains: Metachromasia on toluidine blue staining
- Flow cytometry:
- Positive for ≥2 myeloid-associated markers CD11b, CD13, CD33, CD34, CD38, CD123, CD203c and MPO.
- Usually positive for CD9 and weak CD117.
- Negative: HLA-DR.
- Strong expression of CD117 in abnormal mast cells distinguishes mast cell leukaemia from acute basophilic leukaemia.
- Account for <1% of AML
Acute Myelomonocytic Leukemia (FAB – AML- M4)
- It is acute leukemia characterized by proliferation of both neutrophil and monocyte precursors
- PS/BM show, out of total nucleated cells
- >20% blasts (Including promonocytes, monoblasts and myeloblasts)
- >20% neutrophils and their precursors
- >20% monocyte lineage cells.
- Flow cytometry:
- Positive for myeloid-associated markers (e.g. myeloperoxidase, CD13, CD15, CD33, CD56 and CD117).
- Positive for Monocytic markers (e.g. CD4, CD11b, CD11c, CD14, CD36, CD64, and CD163)
- CD 7 is usullay positive
- Accounts for 15-25% of total AML
Acute Monoblastic (FAB- M5a) and Monocytic Leukemias (FAB- M5b) (Schilling Type)
- These are AMLs in which 80% or more of leukemic cells are of monocytic lineage (Neutrophil component less than 20%)
- Acute Monoblastic Leukemia - > 80% cells are monoblasts (Out of all tumor cells)
- Acute Monocytic Leukemia - > 80% cells are promonocytes (Out of all tumor cells)
- Account for 5 – 8 % of all AMLs
- Erythro / Hemophagocytosis is seen in cases with t (8;16)
- Tissue infiltration is commonly seen. So these patients have
- Gingival hypertrophy
- Ulcerative lesions in rectum and vagina
- Extramedullary masses
- CNS involvement
- Hepatosplenomegaly
- Flow cytometry:
- Positive for myeloid-associated antigens such as CD13, CD15, CD33 (bright), CD65, and CD117.
- Positive for CD34 and usually HLA-DR.
- Positive for 2 or more markers of monocytic differentiation such as CD4 (dim), CD11b, CD11c, CD14, CD36 (bright), CD64 (bright), CD163, LILRB1, and LILRB4).
- Genetics
- Monoblastic leukemia- Translocations involving chromosome 11q23 especially (9:11)
- Monocytic – t (8; 16) (p11; p13) (associated with erythrophagocytosis and coagulopathy)
- Serum lysozyme (muramidase) levels – Elevated
- Prognosis: Poor
Acute erythroid leukemia- (FAB-M6, Di Guglielmo's syndrome)
- Extremely rare and accounts for <1% of all AML.
- >80% cells in bone marrow are erythroid precursors (most of which have dysmorphic morphology)
- >30% are immauture erythroid cells (erythroblasts and pronormoblasts).
- Erythroblasts:
- Medium to large sized cell with a round central nucleus, dispersed to finely reticulated chromatin, one or several distinct nucleoli,
- Cytoplasm is deeply basophilic and agranular. May have blebs and vacuoles.
- Periodic acid-Schiff stain: Coarse globular staining pattern.
- Differential diagnosis- MDS- RAEB, AML with maturation with increased erythroid precursors, AML with multilineage dysplasia, megaloblastic anaemia, haemolytic anemia, megakaryoblastic leukaemia
- Immunophenotyping - Erythroblasts
- Positive: CD36, CD71, and CD117 (often subset). CD235 (Glycophorin A) is positive in a subset of cells that are CD117-negative.
- Aberrantly expressed: CD13, CD4
- Variable: CD45
- Negative: CD34, HLA-DR and myeloid associated markers
- CD117 and E-cadherin positive in erythroblasts at early maturation stages.
- NGS panel: Biallelic (also, multi-hit) TP53 loss of function is characteristic
- Prognosis: Poor
Acute megakaryoblastic leukaemia (FAB- M7)
- Clinical subtypes: Children with Down syndrome, children without Down syndrome, and adults.
- It is an acute leukaemia in which >20% of blasts are of megakaryocyte lineage
- Aspiration is usually dry tap because of myelofibrosis
- Mixture of poorly differentiation blasts and maturing dysplastic megakaryocytes
- In t(1:22) (p13;q13) – Stromal pattern of marrow infiltration mimicking metastatic tumour is seen
- Immunophenotyping- Blasts are positive for CD36, CD41 (glycoprotein IIb), CD42b (glycoprotein Ib), and CD61 (glycoprotein IIIa). Myeloid marker- CD33 is often positive. MPO is negative.
Myeloid sarcoma
(Extramedullary myeloid tumour, granulocytic sarcoma, Chloroma)
- It is a tumour mass of myeloblasts or immature myeloid cells occurring in an extra medullary site
- May precede / seen concurrently with AML/CML/ MDS.
- Sites- Subperiosteum (Skull, sinuses, sternum, ribs, vertebral, pelvis), lymph nodes and skin
- Composed of myeloblasts, neutrophils and neutrophil precursors
- 3 types depending on maturation
- Blastic- Myeloblasts
- Immature- Promyelocytes
- Differentiated- Mature neutrophils
- Immunophenotyping
- Done on sections / Imprint smears
- Similar to AML with / without maturation
- Positive for myeloid associated antigens
- Prognosis: As that of underlying leukaemia
Figures:
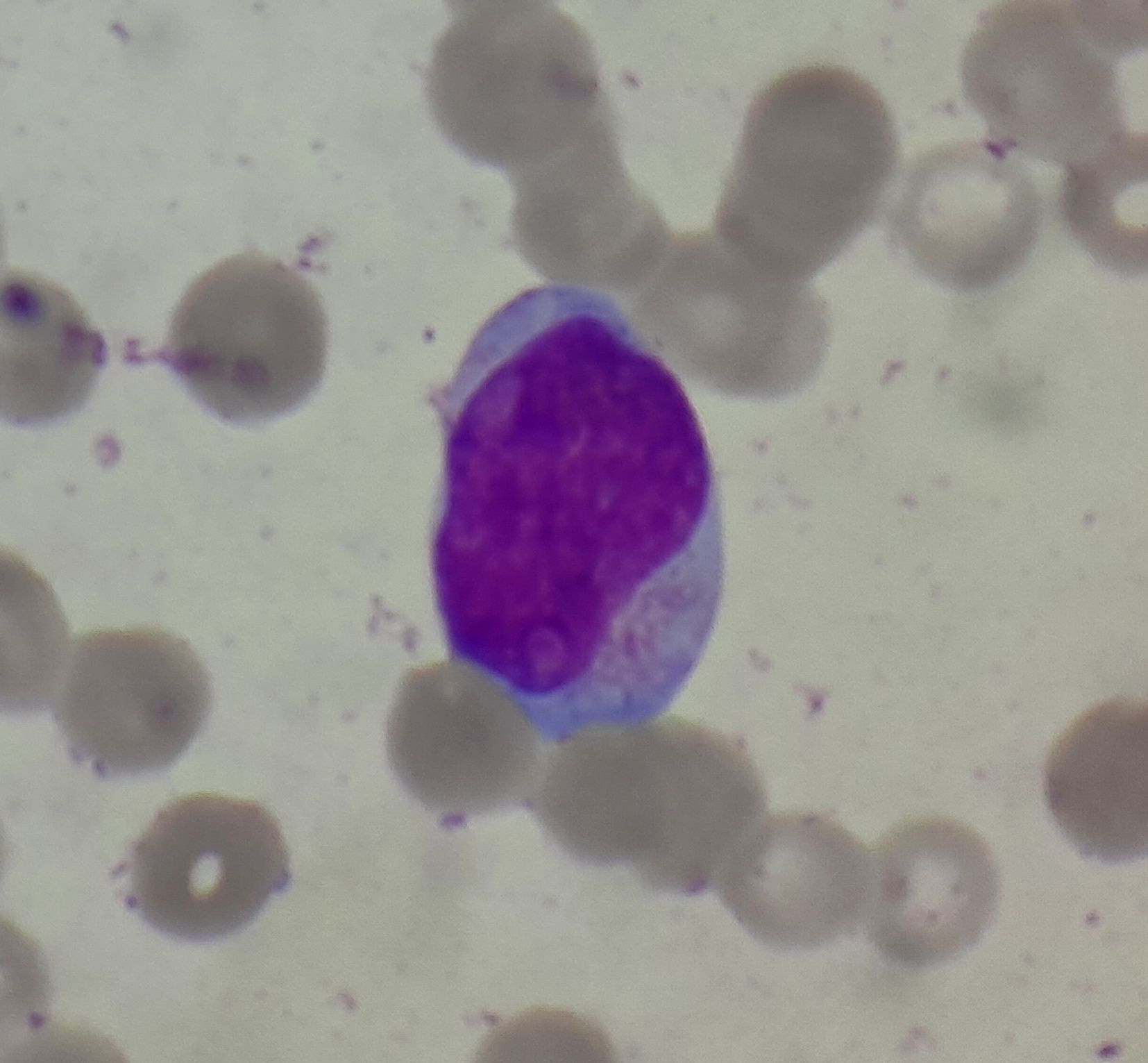
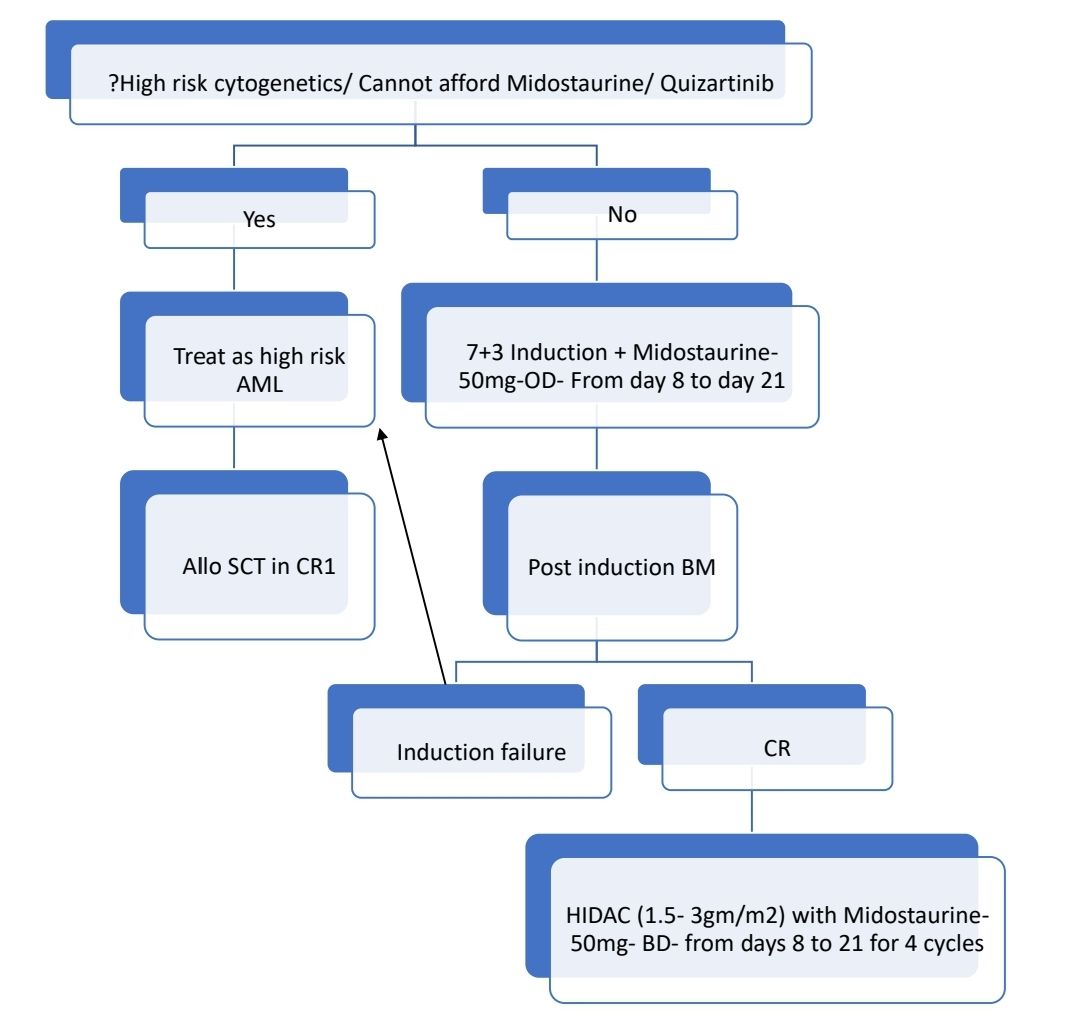
Figure- 4.2.1- Myeloblast without Auer rods
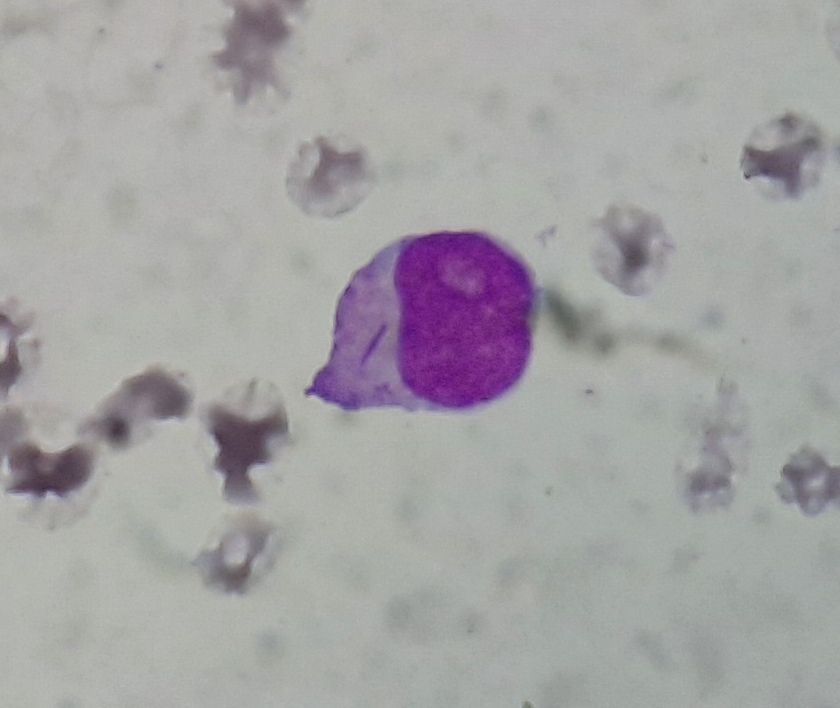
Figure- 4.2.2- Myeloblast with Auer rod
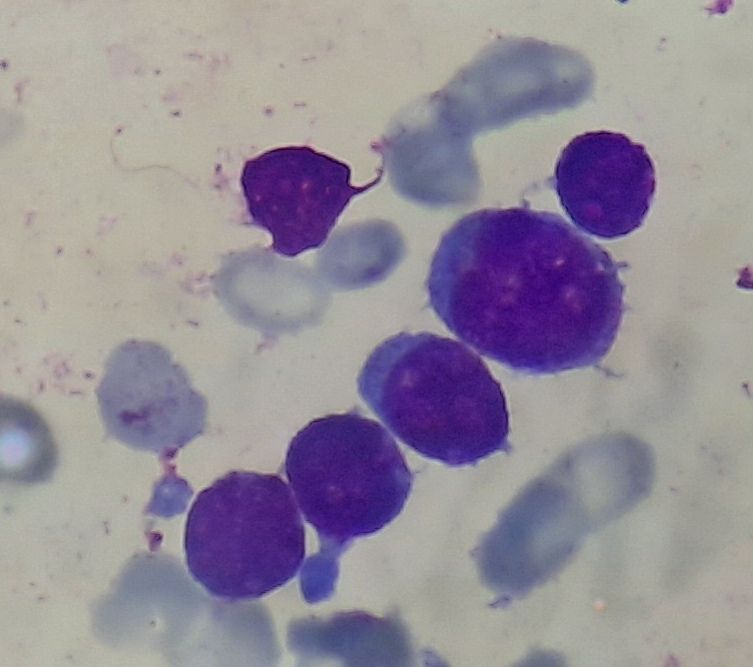
Figure 4.2.4- AML without maturation
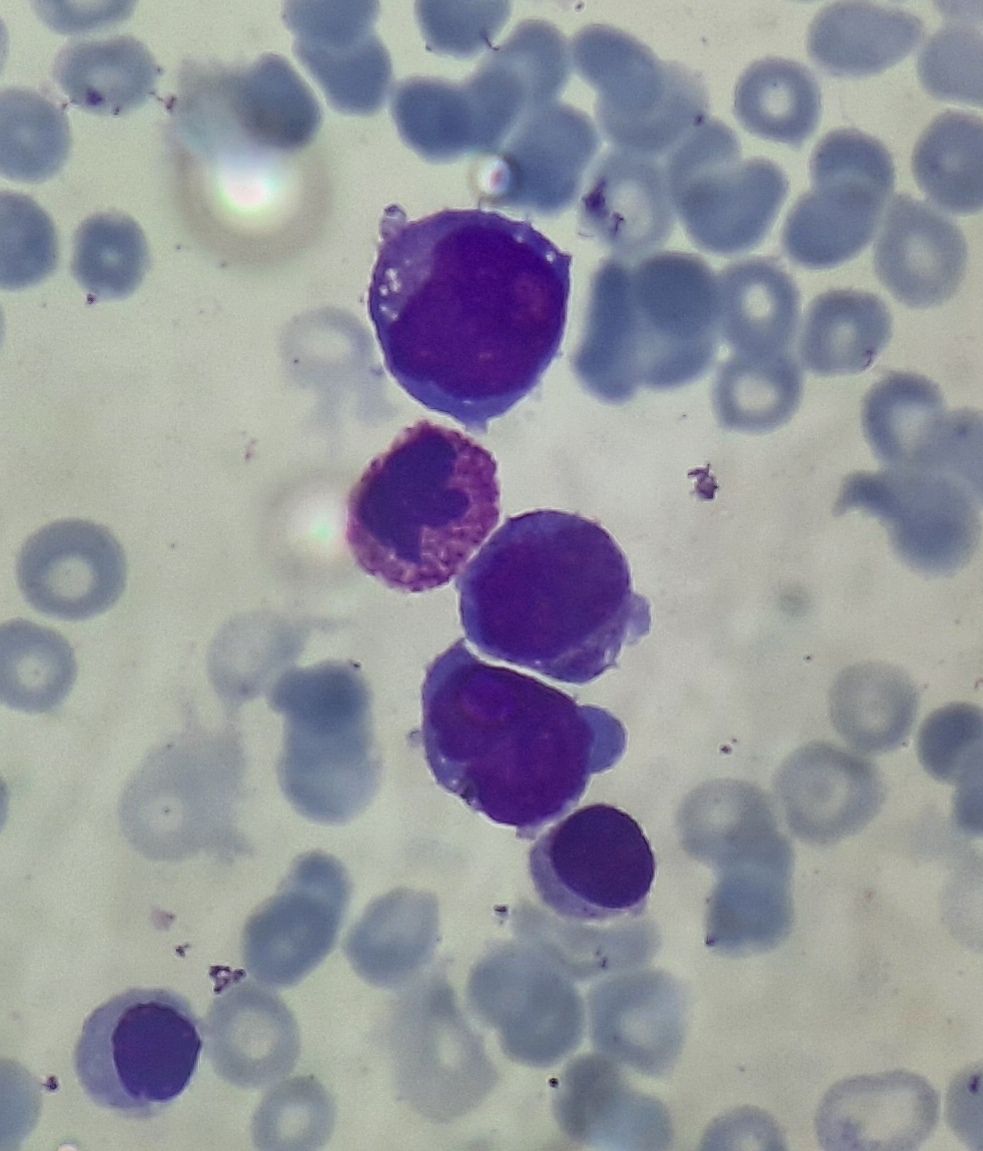
Figure 4.2.5- AML with maturation
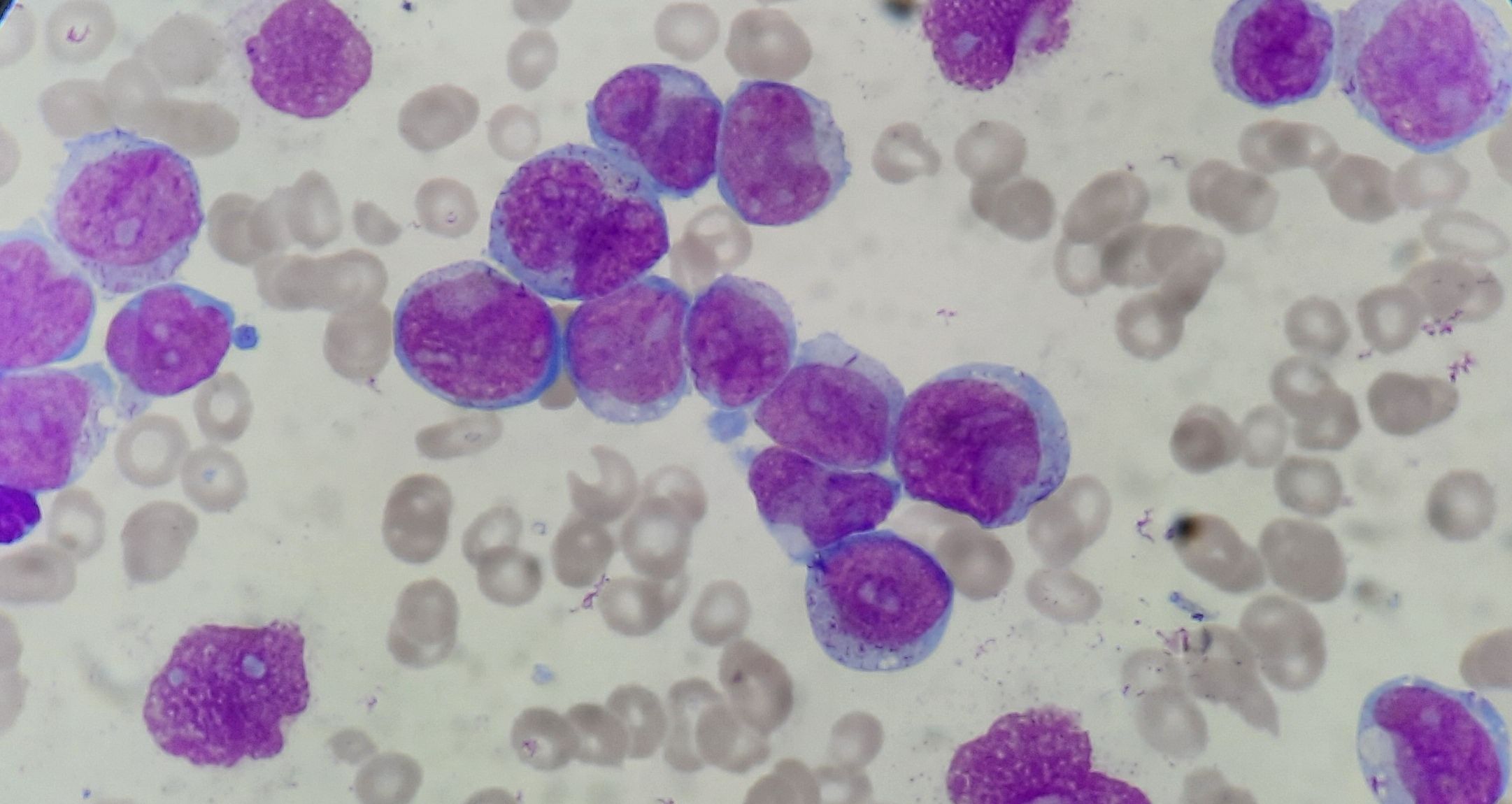
Figure 4.2.6- Acute Monoblastic leukemia
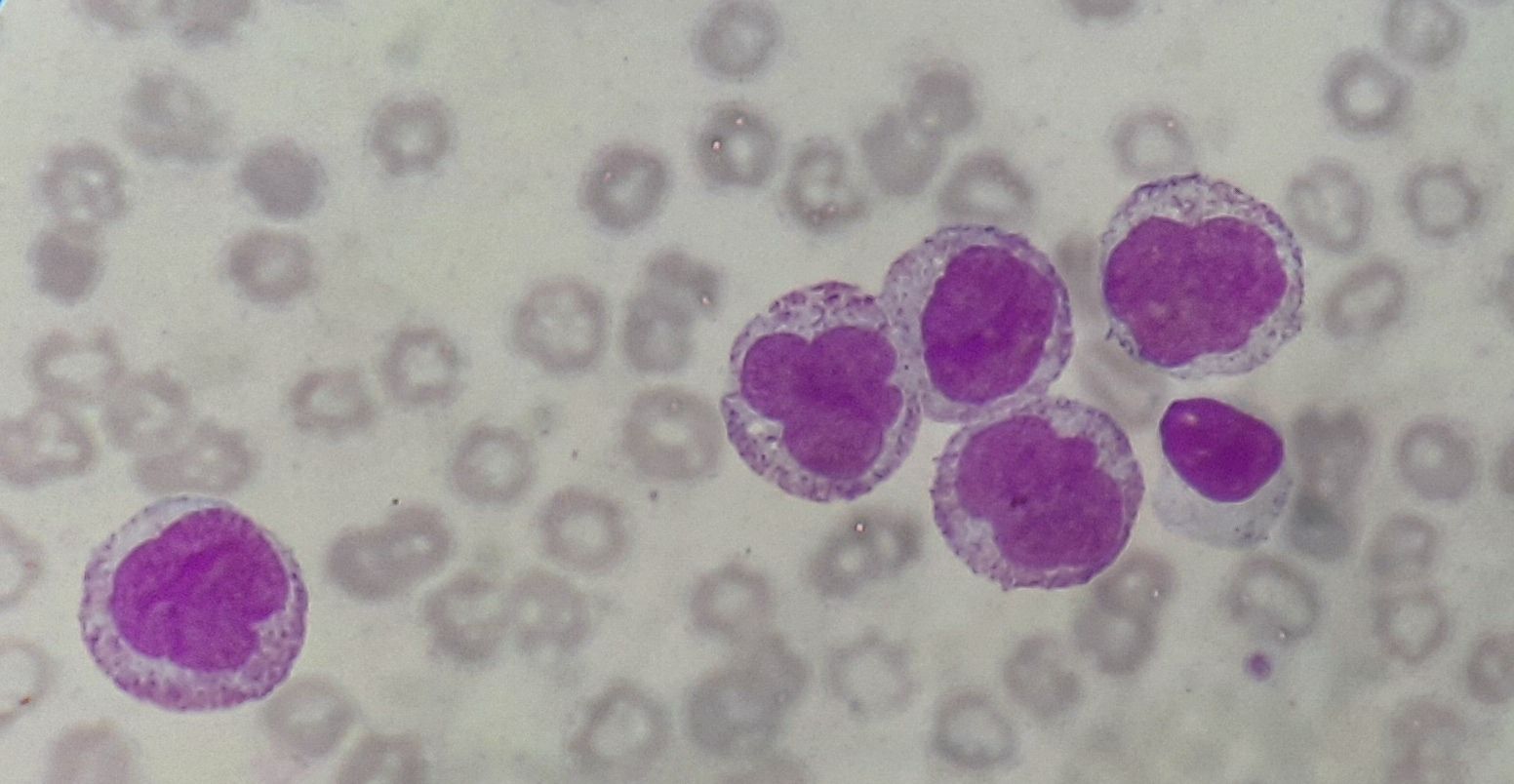
Figure 4.2.7- Acute monocytic leukemia
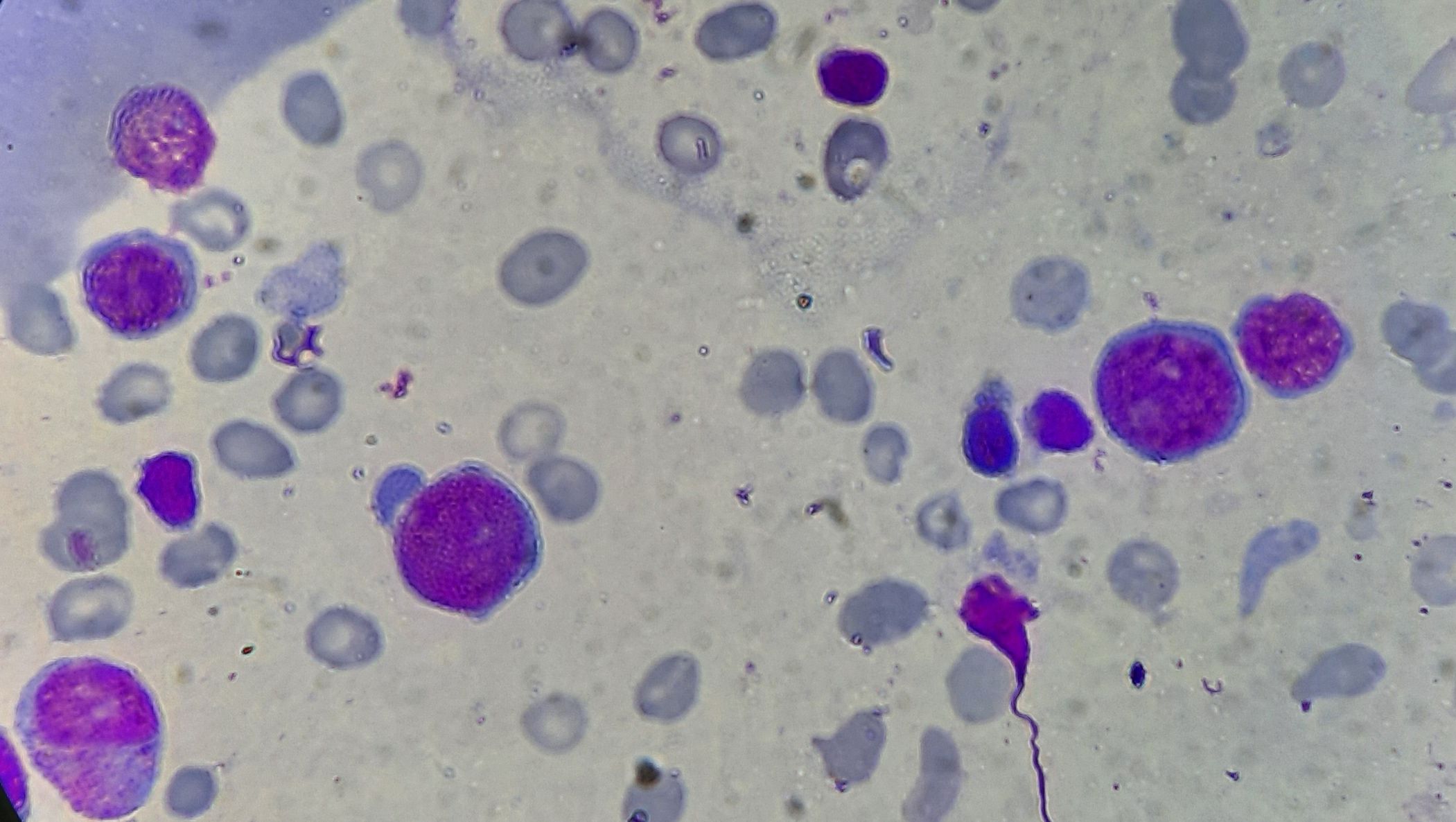
Figure 4.2.8- Acute Erythroleukemia
Recent advances:
Oral Azacytidine Prolongs survival of patients with AML in CR independently of MRD
Recently published phase 3 QUAZAR AML-001 trial results have shown that use of oral azacytidine as post remission maintenance therapy prolongs MRD negativity and also converts MRD+ patients to MRD- status. This trial has also showed that oral azacytidine significantly prolongs overall survival and relapse-free survival. Patients included in this trial had received intensive chemotherapy for remission induction and were not fit enough to undergo hematopoietic stem cell transplantation. ≥0.1% leukemic cells in bone marrow was considered as MRD positivity in the present study.
https://doi.org/10.1182/blood.2021013404
Gilteritinib enhances graft-versus-leukemia effects against FLT3-ITD mutant leukemia after allogeneic hematopoietic stem cell transplantation
Sorafenib, a first generation FLT3 and multikinase inhibitor, was recently shown to enhance graft-versus-leukemia effects against FLT3-ITD+ leukemia via interleukin-15 production. Hence researchers studied the effect of gilteritinib, a selective FLT3 inhibitor in mice models. Bioluminescent imaging using luciferase-transfected Ba/F3-FLT3-ITD demonstrated that gilteritinib significantly suppressed leukemia expansion after allo-SCT, whereas it did not impact the morbidity or mortality of graft-versus-host disease, resulting in significant improvement of overall survival.
https://doi.org/10.1038/s41409-022-01619-4
Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia
Ivosidenib is an inhibitor of mutant isocitrate dehydrogenase 1 (IDH1). It is given in a dose of 500mg- daily. When combined with azacytidine, event-free survival was significantly longer, compared to the placebo-and-azacitidine group. The median overall survival was 24.0 months with ivosidenib and azacitidine and 7.9 months with placebo and azacitidine
https://doi.org/10.1056/NEJMoa2117344
Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia
Ivosidenib is an inhibitor of mutant isocitrate dehydrogenase 1 (IDH1). It is given in a dose of 500mg- daily. When combined with azacytidine, event-free survival was significantly longer, compared to the placebo-and-azacitidine group. The median overall survival was 24.0 months with ivosidenib and azacitidine and 7.9 months with placebo and azacitidine
https://doi.org/10.1056/NEJMoa2117344
Dietary methionine starvation impairs acute myeloid leukemia progression
An amino acid dropout screen on primary leukemic stem cells and progenitor populations revealed a number of amino acid dependencies, of which methionine was one of the strongest. By using various metabolite rescue experiments, nuclear magnetic resonance−based metabolite quantifications and 13C-tracing, polysomal profiling, and chromatin immunoprecipitation sequencing, present study identified that methionine is used predominantly for protein translation by malignant cells of AML. Methionine depletion also reduced total RNA levels, enhanced apoptosis, and induced a cell cycle block.
https://doi.org/10.1182/blood.2022017575
Sorafenib in Childhood Acute Myeloid Leukemia
High allelic ratio FLT3/ITD (AR > 0.4) mutations confer poor prognosis in pediatric acute myeloid leukemia. COG AAML1031 studied the feasibility and efficacy of adding sorafenib, a multikinase tyrosine kinase inhibitor to standard chemotherapy and as single-agent maintenance therapy. Study found that Sorafenib can be safely added to conventional AML chemotherapy and may improve outcomes in pediatric HAR FLT3/ITD+ AML.
https://doi.org/10.1200/JCO.21.01612
Long-Term Benefits of Tagraxofusp for Patients With Blastic Plasmacytoid Dendritic Cell Neoplasm
Present study evaluated CD123-targeted therapy tagraxofusp in adults with treatment-naive and relapsed/refractory BPDCN. In first-line patients, Tagraxofusp monotherapy resulted in high and durable responses, allowing many to bridge to stem-cell transplant.
https://doi.org/10.1200/JCO.22.00034
Venetoclax and hypomethylating agents yield high response rates and favourable transplant outcomes in patients with newly diagnosed acute myeloid leukaemia
The combination of hypomethylating agent (HMA) azacytidine with venetoclax (HMA+Ven) was compared to azacytidine monotherapy in the recent multicentre phase III VIALE-A trial in patients with newly diagnosed acute myeloid leukaemia (AML) unfit for intensive chemotherapy either due to age (≥75 years) or comorbidities. The composite complete remission (CR) rate was 66·4% versus 28·3% in the study arm versus azacytidine alone with median overall survival of 14·7 versus 9·6 months in favour of the HMA+Ven arm. Present study analysed the clinical outcomes of 51 patients aged ≤74 years treated with HMA+Ven induction and subsequently underwent HCT in remission. A total of 25 patients (49%) eventually proceeded to allo-HCT after induction with HMA+Ven. All patients received reduced-intensity conditioning with fludarabine and melphalan and received peripheral blood stem cells grafts. In 41% patients who underwent allo-HCT, the NRM at day 100 was 12% and the 1-year LFS/OS was 67%/85% This study suggests that HMA+Ven may be an effective regimen prior to transplant in patients with AML.
https://doi.org/10.1111/bjh.17996
Enasidenib vs conventional care in older patients with late-stage mutant-IDH2 relapsed/refractory AML
Enasidenib, is an oral IDH2 (isocitrate dehydrogenase 2) inhibitor. In the present study, which included patients aged ≥60 years with late-stage, mutant-IDH2 acute myeloid leukemia relapsed/refractory to 2 or 3 prior AML-directed therapies, 319 patients were randomized to enasidenib or conventional care regimens. Enasidenib provided meaningful benefits in EFS, TTF, ORR, HI, and RBC-TI in this heavily pretreated older mutant-IDH2 R/R AML population.
https://doi.org/10.1182/blood.2021014901
Venetoclax Plus Gilteritinib for FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia
FLT3 inhibitor gilteritinib is standard therapy for relapsed/refractory FLT3-mutated AML. Present study enrolled patients with FLT3 wild-type and FLT3mut (escalation) or FLT3mut (expansion) relapsed/refractory AML. Patients received 400 mg oral venetoclax once daily and 80 mg or 120 mg oral gilteritinib once daily. The combination of venetoclax and gilteritinib was associated with high mCRc and FLT3 molecular response rates regardless of prior FLT3 inhibitor exposure.
https://doi.org/10.1200/JCO.22.00602
Prognostic Value of FLT3-Internal Tandem Duplication Residual Disease in Acute Myeloid Leukemia
The applicability of FLT3-internal tandem duplications (FLT3-ITD) for assessing measurable residual disease (MRD) in acute myeloid leukemia (AML) in complete remission (CR) has been hampered by patient-specific duplications and potential instability of FLT3-ITD during relapse. Present study comprehensively investigated the impact of next-generation sequencing–based FLT3-ITD MRD detection on treatment outcome in a cohort of patients with newly diagnosed AML. Study showed that NGS-based detection of FLT3-ITD MRD in CR identifies patients with AML with profound risk of relapse and death that outcompetes the significance of most established prognostic factors at diagnosis and during therapy.
https://doi.org/10.1200/JCO.22.00715
Characterisation of infections in patients with acute myeloid leukaemia receiving venetoclax and a hypomethylating agent
Present retrospective, multicentre cohort study investigated the incidence of invasive fungal infections and other infectious complications in patients receiving venetoclax and hypomethylating agent therapy for acute myeloid leukaemia. Among 235 patients, the incidence of probable or confirmed invasive fungal infection was 5.1%. The overall incidence of developing at least one bacterial infection was 33.6%.
https://doi.org/10.1111/bjh.18051
Ipilimumab plus decitabine for patients with MDS or AML in posttransplant or transplant-naïve settings
Present study was done to determine if combining decitabine with ipilimumab (CTLA-4 blocker) could augment responses without causing unacceptable immune toxicity. This study included patients with relapsed, refractory (R/R), or secondary myelodysplastic syndromes (MDS)/acute myeloid leukemia (AML) in both the post-HSCT and transplant-naïve settings. The most frequent grade ≥3 treatment-emergent adverse events were cytopenias. This combination therapy showed a meaningful clinical activity
https://doi.org/10.1182/blood.2022017686
Overlapping features of therapy-related and de novo NPM1-mutated AML
This study compared the genetics, transcriptional profile, and clinical outcomes of therapy-related NPM1-mutated acute myeloid leukemia (t-NPM1 AML), de novo NPM1-mutated AML (dn-NPM1 AML), and therapy-related AML (t-AML) with wild-type NPM1. The findings indicate that t-NPM1 AML and dn-NPM1 AML have similar characteristics and should be considered as a single disease entity, with better overall survival and relapse-free survival compared to t-AML.
https://doi.org/10.1182/blood.2022018108
Sorafenib maintenance after allogeneic haemopoietic stem-cell transplantation in patients with FLT3-ITD Positive AML
In this phase 3 trial conducted across seven hospitals in China, patients with FLT3 internal tandem duplication (FLT3-ITD) acute myeloid leukaemia (AML) who underwent allogeneic hematopoietic stem-cell transplantation (HSCT) were randomly assigned to receive sorafenib maintenance or non-maintenance (control) treatment. The 5-year follow-up data revealed that sorafenib maintenance led to improved overall survival (72.0% vs 55.9%), leukaemia-free survival (70.0% vs 49.0%), and graft-versus-host disease (GVHD)-free, relapse-free survival (58.0% vs 39.2%), along with a lower cumulative incidence of relapse (15.0% vs 36.3%) without increased non-relapse mortality.
https://doi.org/10.1016/S2352-3026(23)00117-5
Venetoclax as post-transplant maintenance therapy for AML
In this study, researchers administered single-agent venetoclax (ven) maintenance to 49 high-risk AML patients from February 2019 to December 2021. Ven was intended for use until 1 year post-SCT, with most patients (88%) completing the full year despite temporary interruptions. Common side effects included cytopenias (40.8%) and gastrointestinal issues (34.7%). At the 1-year post-SCT mark, overall survival (OS) and relapse-free survival (RFS) rates were 70% and 67% respectively. This experience suggests that venetoclax is a well-tolerated and feasible maintenance therapy, potentially enhancing OS and RFS in high-risk post-SCT AML patients.
https://doi.org/10.1038/s41409-023-01987-5
Influenza A (H1N1) virus induced long-term remission in a refractory acute myeloid leukaemia
This study reports a unique case of a refractory acute myeloid leukemia (AML) patient achieving long-term complete remission (CR) following infection with the influenza A virus (IAV, H1N1 subtype). The patient displayed increased proportions of helper T cells after IAV infection, and higher levels of cytokines such as IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ, and TNF-α were detected in IAV-infected patients compared to control groups. This suggests that IAV-induced anti-tumor effects may be linked to immune response modification. The study presents new clinical evidence of IAV's potential anti-tumor effects.
https://doi.org/10.1111/bjh.18876
Gilteritinib in Patients With Newly Diagnosed AML
Gilteritinib, a type 1 FLT3 inhibitor, was investigated in a phase IB study for its safety, tolerability, and efficacy when integrated into intensive induction and consolidation chemotherapy, as well as maintenance therapy for adults with newly diagnosed, non-favorable-risk AML. After dose escalation, a daily dose of 120 mg gilteritinib was chosen for further study. In patients with FLT3-mutated AML, the composite complete response rate was 89%, with a median overall survival of 46.1 months. Gilteritinib was generally well-tolerated, though time to count recovery during induction was longer, associated with higher gilteritinib levels, and influenced by azole use.
https://doi.org/10.1200/JCO.22.02721
Allogeneic hematopoietic cell transplantation for patients with AML aged 70 years or older in first remission
This study investigated the outcomes of 701 adults aged ≥70 years with acute myeloid leukemia (AML) in first complete remission (CR1) who received their first hematopoietic cell transplantation (HCT) from various donor sources. The 2-year overall survival (OS) was 48.1%, leukemia-free survival (LFS) was 45.3%, relapse incidence (RI) was 25.2%, non-relapse mortality (NRM) was 29.5%, and GVHD-free, relapse-free survival (GRFS) was 33.4%. Compared to HLA-matched sibling donors (MSD), patients from haploidentical (Haplo) and HLA-matched unrelated donors (UD) had lower RI, resulting in prolonged LFS for Haplo. Patients from 9/10 HLA-mismatched unrelated donors (mUD) had the highest NRM incidence. The study suggests that HCT in selected AML patients aged >70 years in CR1 is feasible and associated with favorable outcomes, warranting further prospective clinical trials.
https://doi.org/10.1038/s41409-023-02027-y
FLT3-directed UniCAR T-cell therapy of acute myeloid leukaemia
Adaptor chimeric antigen receptor (CAR) T-cell therapy is emerging as a promising approach to address safety and antigen escape issues in myeloid malignancies. The 'UniCAR' platform is currently in early clinical investigation and has demonstrated its potential in treating acute myeloid leukemia (AML) by offering a rapidly switchable CD123-directed UniCAR T-cell product. However, given the plasticity of relapsed and refractory AML, targeting multiple tumor antigens is crucial for sustained anti-tumor responses. This study presents the preclinical development of a novel UniCAR T-cell therapy directed at FMS-like tyrosine kinase 3 (FLT3), showing high effectiveness in killing AML cell lines and primary AML samples in vitro, as well as in vivo functionality in a murine model. PET analyses reveal a short serum half-life of FLT3 target modules, enabling a rapid on/off switch of UniCAR T cells. These promising preclinical findings support further development and clinical translation of FLT3-specific UniCAR T cells for AML therapy.
https://doi.org/10.1111/bjh.18971
Molecular targeting of the UDP-glucuronosyl transferase enzymes in high-eukaryotic translation initiation factor 4E refractory/relapsed acute myeloid leukemia patients
In a phase II trial, the combination of vismodegib with ribavirin, with or without decitabine, was explored in heavily pre-treated patients with high-eIF4E acute myeloid leukemia (AML). Elevated UDP-glucuronosyltransferase 1A (UGT1A) levels, associated with glucuronidation activity, were observed in patients' blasts. Vismodegib, which inhibits GLI1 and reduces UGT1A levels, showed promise in patients with partial response, blast response, or prolonged stable disease, indicating effective targeting of eIF4E by ribavirin. These findings suggest that UGT1A protein and glucuronidation are targetable in humans, opening avenues for therapies that mitigate drug deactivation through glucuronidation.
https://doi.org/10.3324/haematol.2023.282791
10-day decitabine versus 3 + 7 chemotherapy followed by allografting in older patients with acute myeloid leukaemia
In a phase 3 trial involving older patients (aged 60 and above) with newly diagnosed acute myeloid leukaemia (AML), decitabinemonotherapy was compared to standard chemotherapy (3+7 regimen). The trial included 606 patients and found that the 10-day decitabine treatment did not improve overall survival compared to the 3+7 chemotherapy. However, decitabine demonstrated a better safety profile with lower rates of certain adverse events, suggesting that it could be considered as a better-tolerated alternative to standard chemotherapy in fit older patients with AML who are not eligible for allogeneic hematopoietic stem-cell transplantation.
https://doi.org/10.1016/S2352-3026(23)00273-9
Cusatuzumab plus azacitidine in newly diagnosed acute myeloid leukaemia ineligible for intensive chemotherapy (CULMINATE)
In this phase 2, dose-optimization study, cusatuzumab, a high-affinity anti-CD70 antibody, was investigated in combination with azacitidine for the treatment of previously untreated acute myeloid leukemia (AML) patients who are not eligible for intensive chemotherapy. Two dose cohorts, 10 mg/kg and 20 mg/kg of cusatuzumab, were evaluated. The complete remission rate was 12% for the 10 mg/kg group and 27% for the 20 mg/kg group. Although the study was not designed for a formal comparison, the data suggested that cusatuzumab 20 mg/kg plus azacitidine might be the optimal dose for further studies.
https://doi.org/10.1016/S2352-3026(23)00207-7
Sorafenib plus intensive chemotherapy in newly diagnosed FLT3-ITD AML
In a placebo-controlled, phase 2 study involving 102 patients with FMS-like tyrosine kinase 3–internal tandem duplication (FLT3-ITD) acute myeloid leukemia (AML), the addition of sorafenib to intensive induction chemotherapy did not significantly improve event-free survival (EFS) compared to placebo (2-year EFS 47.9% vs. 45.4%). However, the 2-year overall survival (OS) was higher in the sorafenib arm (67% vs. 58%), and in patients who received hematopoietic cell transplant in first remission, the 2-year OS rates were 84% in the sorafenib arm compared to 67% in the placebo arm. FLT3-ITD measurable residual disease negative status after induction was associated with improved 2-year OS.
https://doi.org/10.1182/blood.2023020301
Pediatric acute myeloid leukemia and hyperleukocytosis with WBC count greater than 50 × 109/L
In a study investigating the impact of hyperleukocytosis (defined as WBC ≥ 50 × 10^9/L) on the prognosis of pediatric acute myeloid leukemia (AML), 27.4% of AML patients exhibited hyperleukocytosis. Patients with hyperleukocytosis had similar complete remission (CR) and overall survival (OS) rates to those without hyperleukocytosis but had a lower event-free survival (EFS) rate. Among patients with hyperleukocytosis, the study found that pediatric AML patients had a similar prognosis regardless of whether their WBC count was 50–100 × 10^9/L or ≥ 100 × 10^9/L. The FAB M5 subtype was associated with significantly inferior survival, while the prognosis of CBF-AML was good.
https://doi.org/10.1007/s12185-023-03665-0
Autologous stem cell transplantation in adult patients with intermediate-risk acute myeloid leukemia
The study investigated the choice of consolidation therapy in patients with intermediate-risk (IR) acute myeloid leukemia (AML) in first remission (CR1) with no measurable residual disease (MRD negative). The analysis included 1,122 adult patients transplanted between 2010 and 2021, with 547 receiving autologous stem cell transplantation (ASCT) and 575 receiving a haploidentical donor transplant. The comparisons were stratified based on FLT3 mutation status (FLT3-wt for wild-type and FLT3-ITD for mutation). In FLT3-wt patients, haploidentical transplants had lower relapse incidence (RI), higher non-relapse mortality (NRM), similar leukemia-free survival (LFS), and lower overall survival (OS) compared to ASCT. In FLT3-ITD patients, haploidentical transplants had lower RI, higher NRM, better LFS, and similar OS compared to ASCT. The study suggests that autologous transplantation is a valid option for FLT3-wt patients, while haploidentical transplant is preferable for FLT3-ITD patients in MRD-negative CR1.
https://doi.org/10.1038/s41409-023-02070-9
Fludarabine, cytarabine, and idarubicin with or without venetoclax in patients with relapsed/refractory acute myeloid leukemia
The study compared the safety and efficacy of fludarabine, cytarabine, and idarubicin (FLA-IDA) with or without venetoclax (FLAVIDA) in patients with relapsed and refractory acute myeloid leukemia (R/R AML). The overall response rate (ORR) was significantly higher in FLAVIDA compared to FLAIDA-treated patients. Measurable residual disease was negative at a similar proportion in responding patients in both groups. Patients in both groups showed similar rates of proceeding to allogeneic hematopoietic cell transplantation or donor lymphocyte infusion. Event-free and overall survival were similar between FLAVIDA- and FLA-IDA-treated patients.
https://doi.org/10.3324/haematol.2023.282912
Standard versus high-dose cytarabine with or without vorinostat for AML
The S1203 multicenter trial investigated the use of higher-dose cytarabine during induction therapy for previously untreated acute myeloid leukemia (AML). The study compared daunorubicin and cytarabine (DA), idarubicin with higher-dose cytarabine (IA), and IA with vorinostat (IA + V) in patients aged 18–60. The overall remission rate was 77.5%, with no significant differences in outcomes among the three arms. The favorable cytogenetics subset showed improved outcomes with DA and post-remission high-dose cytarabine. The study concluded that higher-dose cytarabine, with or without vorinostat, did not result in improved outcomes in younger AML patients.
https://doi.org/10.1038/s41375-023-02073-x
Real-world data of AML in Japan
This report presents results from a multicenter, prospective observational study of acute myeloid leukemia (AML), myelodysplastic syndromes, and chronic myelomonocytic leukemia in Japan. The study included 3728 AML patients registered between August 2011 and January 2016. The estimated 5-year overall survival (OS) rate in AML patients was 31.1%, with trial-enrolled patients having a 1.7-fold higher OS rate than non-enrolled patients (58.9% vs. 35.5%, p < 0.0001). Women had a higher OS rate than men (34% vs 27.7%, p < 0.0001), and the OS rate decreased with age, with lower rates in patients aged 40 and older (5-year OS for ages < 40, 40–64, 65–74, ≥ 75: 74.5% vs 47.5% vs 19.3% vs 7.3%, respectively). This study provides large-scale data on survival and clinical characteristics in Japanese AML patients.
https://doi.org/10.1007/s12185-023-03677-w
Long-term follow-up of haploidentical haematopoietic stem cell transplantation in paediatric patients with high-risk acute myeloid leukaemia
The study analyzed data from 200 children with high-risk acute myeloid leukemia who underwent their first haploidentical hematopoietic stem cell transplantation (haplo-HSCT) between 2015 and 2021. The 4-year overall survival (OS) was 71.9%, with event-free survival (EFS) at 62.3% and a cumulative relapse incidence (CIR) of 32.4%. Acute graft-versus-host disease (aGVHD) rates were notable, with 100-day cumulative incidences of grade II–IV and III–IV aGVHD at 41.1% and 9.5% respectively, while the 4-year cumulative incidence of chronic GVHD (cGVHD) was 56.1%, with moderate-to-severe cGVHD at 27.3%. Minimal residual disease (MRD) positivity pre-HSCT significantly correlated with lower survival and higher relapse risk.
https://doi.org/10.1111/bjh.19086
Low-dose cytosine with lenalidomide in acute myeloid leukaemia
In the LI-1 trial, lenalidomide (LEN) combined with low-dose cytosine arabinoside (LDAC) was evaluated in patients aged over 60 years unfit for intensive therapy with acute myeloid leukaemia (AML), compared to LDAC alone. The combination therapy showed a higher overall response rate (complete remission + complete remission with incomplete hematological recovery) compared to LDAC alone (26% vs. 13.7%, p = 0.031). However, there was no significant difference in overall survival between the two treatment arms at 2 years (14% for LDAC+LEN vs. 11.5% for LDAC alone). Importantly, the addition of LEN was associated with increased toxicity and supportive care requirements.
https://doi.org/10.1111/bjh.19220
Cardiac events in newly diagnosed acute myeloid leukaemia during treatment with venetoclax + hypomethylating agents
Among 301 newly diagnosed patients with acute myeloid leukemia (AML) treated with venetoclax and a hypomethylating agent, 7.6% experienced major cardiac complications, including cardiomyopathy, non-ST elevation myocardial infarction, and pericarditis/effusions. Baseline characteristics revealed a predominantly older male population with cardiovascular risk factors and preserved baseline ejection fraction. In multivariate analysis, males were more likely and cases with DNMT3A mutations were less likely to experience cardiac complications.
https://doi.org/10.1111/bjh.19325
Characteristics and outcomes of acute myeloid leukaemia patients with baseline CD7 expression
To develop targeted therapies for acute myeloid leukemia (AML), specific expression profiles are crucial. In a study of 901 AML patients, 263 (29.2%) exhibited aberrant CD7 expression on leukemic blasts. CD7+ AML was associated with adverse risk (64.6% vs. 55.6%, p = 0.0074) and less likely to be favorable risk (15.2% vs. 24.1%, p = 0.0074) according to the European LeukemiaNet 2022 criteria. CD7+ AML patients had inferior overall survival (11.9 months [95% CI, 9.7–15.9] vs. 19.0 months [95% CI, 16.1–23.0], p = 0.0174). At relapse, CD7 expression showed moderate instability, with 30.4% losing and 19.0% gaining CD7 expression.
https://doi.org/10.1111/bjh.19446
A 4-gene prognostic index for enhancing acute myeloid leukaemia survival prediction
Despite advancements in genetic markers for predicting acute myeloid leukaemia (AML) outcomes, significant heterogeneity remains. This study assessed the transcriptomes of 975 chemotherapy-treated AML patients across five cohorts, identifying a 4-gene prognostic index (4-PI) including CYP2E1, DHCR7, IL2RA, and SQLE. The 4-PI stratified patients into risk categories, with high 4-PI groups showing TP53 mutations and cholesterol biosynthesis signatures, and leukaemia stem cell enrichment identified via single-cell RNA sequencing. Validation in three additional cohorts (n = 671) confirmed its reproducibility and clinical utility, outperforming 56 established prognostic indexes and refining risk stratification, particularly reclassifying intermediate ELN2017 category patients to adverse risk. The 4-PI is a robust, straightforward tool for improving AML survival predictions.
https://doi.org/10.1111/bjh.19472
Identification of a very poor prognosis genetic group in acute myeloid leukaemia patients undergoing allogeneic haematopoietic stem cell transplantation
The European LeukemiaNet (ELN) 2022 risk classification for acute myeloid leukemia (AML) was retrospectively evaluated in 120 patients undergoing allogeneic hematopoietic cell transplantation (allo-HCT), including 99 in first complete response (CR1) from 2011 to 2021. Adverse risk patients showed significantly inferior overall survival (OS) and leukemia-free survival (LFS) outcomes compared to other risk groups. A subgroup termed adverse-plus (AdvP), encompassing complex karyotype, MECOM(EVI1) rearrangements, and TP53 mutations, demonstrated even worse outcomes than the broader adverse risk group.
https://doi.org/10.1111/bjh.19518
Iadademstat in combination with azacitidine in patients with newly diagnosed acute myeloid leukaemia
The ALICE study investigated iadademstat, an LSD1 inhibitor, combined with azacitidine for treating newly diagnosed acute myeloid leukemia (AML) in patients ineligible for intensive chemotherapy. Conducted at six hospitals in Spain, the phase 2a trial enrolled 36 patients with a median age of 76. The recommended phase 2 dose was determined to be 90 μg/m² iadademstat daily with 75 mg/m² azacitidine. The combination showed a manageable safety profile, with common adverse events including decreased platelet and neutrophil counts. The study reported promising efficacy, with 82% of patients showing an objective response, including 52% achieving complete remission.
https://doi.org/10.1016/S2352-3026(24)00132-7
Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia
The final analysis of the VIALE-A study confirms the long-term efficacy and safety of venetoclax-azacitidine for patients with newly diagnosed acute myeloid leukemia (AML) ineligible for intensive chemotherapy. With a median follow-up of 43.2 months, venetoclax-azacitidine significantly improved overall survival (OS) compared to placebo-azacitidine, with a median OS of 14.7 months versus 9.6 months, respectively, and a 24-month OS rate of 37.5% versus 16.9%.
https://doi.org/10.1002/ajh.27246
Hypomethylating agents plus venetoclax compared with intensive induction chemotherapy regimens in molecularly defined secondary AML
Molecularly defined secondary acute myeloid leukemia (AML) linked to prior myeloid neoplasms carries a poorer prognosis. In a study of 395 such patients treated with daunorubicin and cytarabine (7+3), liposomal daunorubicin and cytarabine (CPX-351), or hypomethylating agents (HMA) plus venetoclax (VEN), median overall survival (OS) was similar among patients aged over 60 years. Multivariable analysis showed that HMA+VEN treatment was associated with better OS compared to 7+3 (HR 0.64, p=0.041), while CPX-351 was not (HR 0.79, p=0.31). Certain mutations influenced outcomes: BCOR and IDH mutations improved OS, whereas older age, prior myeloid disease, NRAS/KRAS mutations, EZH2 mutation, and monosomal karyotype worsened OS.
https://doi.org/10.1038/s41375-024-02175-0
Gemtuzumab ozogamicin plus midostaurin in combination with standard ‘7 + 3’ induction therapy in newly diagnosed AML
In this phase I trial for newly diagnosed acute myeloid leukemia (AML), the combination of gemtuzumab ozogamicin (GO) and midostaurin with intensive chemotherapy was investigated in patients with FLT3-mutated AML and core-binding factor (CBF) leukemia. Three dose levels of midostaurin and one to three sequential doses of 3 mg/m2 GO with '7 + 3' induction chemotherapy were evaluated. Safety findings in 12 patients indicated that 3 mg/m2 GO on Days 1 and 4, and 100 mg midostaurin on Days 8–21 can be safely combined with intensive chemotherapy in newly diagnosed AML.
https://doi.org/10.1111/bjh.19436
Peripheral blood stem cell versus bone marrow graft for patients ≥60 years undergoing reduced intensity conditioning haploidentical transplantation for acute myeloid leukemia in complete remission
In a study comparing peripheral blood (PB) versus bone marrow (BM) grafts for T-cell replete haploidentical stem cell transplantation (Haplo-SCT) using post-transplantation cyclophosphamide (PT-Cy), 804 patients over 60 years old with acute myeloid leukemia (AML) in remission were analyzed. The PB group (n = 595) showed a significantly higher incidence of acute GVHD compared to BM (n = 209). However, the PB group exhibited lower risks of relapse and better leukemia-free survival, with a trend towards improved overall survival. These findings suggest that PB grafts may be advantageous in Haplo-SCT with PT-Cy for older AML patients, potentially reducing relapse rates and improving clinical outcomes despite the higher risk of acute GVHD.
https://doi.org/10.1002/ajh.27343
Clinical prognostic value of different NPM1 mutations in acute myeloid leukemia patients
This study investigated the prognostic impact of different NPM1 mutation types in acute myeloid leukemia (AML) patients. Among 528 newly diagnosed AML patients, 25.2% had NPM1 mutations, with 83.5% being type A. Event-free survival (EFS) differed significantly between patients with low variant allele frequency (VAF) of non-A-type and A-type NPM1 mutations. Additionally, overall survival (OS) and EFS were notably different depending on the presence of FLT3-ITD mutations alongside NPM1 mutation types. Although no significant difference was found between A-type and non-A-type NPM1 mutations alone, the combination of NPM1 mutation types and FLT3-ITD status appears to influence prognosis.
https://doi.org/10.1007/s00277-024-05786-w
Cladribine plus cytarabine plus venetoclax in acute myeloid leukemia relapsed or refractory to venetoclax plus hypomethylating agent
The study explored the use of the cladribine, low-dose cytarabine (Ara-C), and venetoclax (CAV) regimen in acute myeloid leukemia (AML) patients who failed venetoclax plus hypomethylating agent (Ven-HMA) therapy. Of 39 patients analyzed, 28% achieved complete remission (CR) or CR with incomplete blood count recovery (CRi). Predictors of response included de novo AML, absence of adverse cytogenetics, and absence of K/NRAS mutations. While CR/CRi rates were lower compared to treatment-naïve settings (28% vs. 93%), CAV provided a valuable salvage option, with median survival of 4.7 months.
https://doi.org/10.3324/haematol.2024.284962
Decitabine in older patients with AML: quality of life results
This phase 3 randomized trial compared health-related quality of life (HRQoL) outcomes in fit older patients with acute myeloid leukemia (AML) treated with decitabine (DEC) versus intensive chemotherapy (IC). DEC showed a lower risk of HRQoL deterioration at 2 months (76% vs 88%), and long-term differences were not statistically significant. HRQoL deteriorated after allogeneic hematopoietic stem cell transplantation (allo-HSCT) in both arms but was less clinically meaningful in the DEC group. These findings suggest DEC may be a preferable option over IC for older AML patients.
https://doi.org/10.1182/blood.2023023625
Prognostic impact of CEBPA mutational subgroups in adult AML
A pooled primary data analysis of 1010 CEBPA-mutant adult AML patients revealed significant differences in outcomes based on the type of mutation and allelic state. Specifically, patients with bZIPInDel mutations exhibited higher rates of complete remission and longer relapse-free and overall survival compared to other CEBPA-mutant subgroups. Co-mutations in bZIPInDel patients did not independently impact overall survival, whereas in non-bZIPInDel patients, co-mutations provided significant prognostic information. These findings suggest that bZIPInDel mutations are the major independent determinant of outcome in CEBPA-mutant AML, refining current classifications according to WHO and ELN2022 recommendations.
https://doi.org/10.1038/s41375-024-02140-x
Azacitidine, Venetoclax, and Gilteritinib in Newly Diagnosed and Relapsed or Refractory FLT3-Mutated AML
This phase I/II study evaluated the combination of azacitidine, venetoclax, and gilteritinib in two cohorts of patients with FLT3-mutated AML, including those who were newly diagnosed and those with relapsed/refractory disease. The recommended phase II dose of gilteritinib was determined to be 80 mg once daily. In the newly diagnosed cohort, the CR/CRi rate was 96%, with encouraging rates of relapse-free survival (RFS) and overall survival (OS). In the relapsed/refractory cohort, the CR/CRi rate was 27%, with additional patients achieving a morphologic leukemia–free state. The most common grade 3 or higher adverse events were manageable, with infection and febrile neutropenia being more frequent in the relapsed/refractory cohort.
https://doi.org/10.1200/JCO.23.019
Crenolanib and Intensive Chemotherapy in Adults With Newly Diagnosed FLT3-Mutated AML
This trial evaluated the efficacy and safety of crenolanib, a second-generation tyrosine kinase inhibitor, combined with intensive chemotherapy in adults with newly diagnosed FLT3-mutant AML. The study included 44 patients, aged 19-75, with 36 having FLT3-ITD and 11 with FLT3-TKD mutations. The treatment regimen achieved an overall response rate of 86%, with a complete remission (CR) rate of 77% and a median event-free survival of 44.7 months. Younger patients had an estimated 3-year survival of 71.4% and a 15% cumulative incidence of relapse. Common serious adverse events were febrile neutropenia, diarrhea, and nausea.
https://doi.org/10.1200/JCO.23.0106
Targeting Molecular Measurable Residual Disease and Low-Blast Relapse in AML With Venetoclax and Low-Dose Cytarabine
This phase II study investigated the safety and efficacy of venetoclax combined with low-dose cytarabine (LDAC) in AML patients with first measurable residual disease (MRD) or oligoblastic relapse. Forty-eight adults with either MRD or oligoblastic relapse participated, receiving venetoclax 600 mg daily and LDAC for 10 days in 28-day cycles. Among MRD relapse patients, a 69% log10 reduction in MRD was seen by cycle 2, with 46% achieving MRD-negative remission, while 73% of the oligoblastic relapse cohort achieved complete remission. Overall, 44% underwent hematopoietic cell transplantation, and the 2-year overall survival rate was 67% for MRD relapse and 53% for oligoblastic relapse. The combination therapy was well-tolerated and highly effective in these AML patient cohorts.
https://doi.org/10.1200/JCO.23.01
Mitoxantrone Versus Liposomal Daunorubicin in Induction of Pediatric AML With Risk Stratification Based on Flow Cytometry Measurement of Residual Disease
This study in pediatric AML used intensified, response-guided induction therapy and MRD-based risk stratification, treating poor induction response with hematopoietic stem-cell transplant (hSCT). The study compared the efficacy of liposomal daunorubicin (DNX) and mitoxantrone in induction therapy. Although initially planned for 300 patients, only 194 were randomly assigned due to DNX production cessation, with an additional 93 non-randomly assigned patients as an observation cohort. The study found no significant difference in MRD levels after induction 1 between the two drugs, but mitoxantrone showed superior outcomes, including higher event-free survival (EFS5y) and lower cumulative incidence of relapse (CIR), particularly in standard-risk patients. High-risk patients had favorable outcomes with hSCT, demonstrating the effectiveness of the intensified induction and risk-based stratification approach.
https://doi.org/10.1200/JCO.23.0184
Gilteritinib with or without venetoclax for relapsed/refractory FLT3-mutated acute myeloid leukaemia
In patients with relapsed/refractory (R/R) FLT3-mutated acute myeloid leukemia (AML), adding venetoclax to gilteritinib (gilt-ven) did not significantly improve remission or rates of allogeneic hematopoietic stem-cell transplantation (HSCT) compared to gilteritinib alone. However, a trend toward better overall survival (OS) was observed with gilt-ven, especially when used early as a salvage approach. The gilt-ven combination was associated with increased hematological toxicity.
https://doi.org/10.1111/bjh.19548
Oral decitabine/cedazuridine versus intravenous decitabine for acute myeloid leukaemia
This study demonstrates the equivalence of oral decitabine with cedazuridine (DEC-C) to intravenous decitabine (DEC-IV) in patients with acute myeloid leukemia (AML) ineligible for intensive chemotherapy. Both formulations showed nearly identical systemic exposures, similar clinical responses, and consistent safety profiles. Median overall survival and demethylation rates were also comparable. The findings support DEC-C as a convenient oral alternative to DEC-IV, offering comparable efficacy and tolerability.
https://doi.org/10.1111/bjh.19741
Prognostic significance of GATA2 in patients with MDS/AML
GATA2 deficiency syndrome, linked to a high risk of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), was analyzed in this meta-analysis to clarify the prognostic significance of GATA2 mutations in MDS/AML. From 13 cohort studies involving 2714 patients (644 with GATA2 mutations), the mutations were associated with poorer overall survival (HR = 1.54) and event-free survival (HR = 1.32), but no significant impact on disease-free survival. GATA2 mutations had a particularly adverse effect on overall survival in MDS patients (HR = 2.56) but not in AML patients (HR = 1.08). These findings suggest that patients with GATA2 mutations, especially in MDS, may benefit from aggressive therapeutic strategies.
https://doi.org/10.1007/s00277-024-05899-2
Overall survival in TP53-mutated AML and MDS
A retrospective review of 210 patients with AML or MDS and TP53 mutations, classified using the 2022 WHO and ICC criteria, revealed significant insights into prognosis. Multi-hit TP53 mutations predicted poorer overall survival (OS) in MDS but not AML under both classification systems. The strongest predictors of OS across all patients were thrombocytopenia (platelet count < 50 K; HR: 2.01) and a TP53 variant allele frequency (VAF) ≤ 40% (HR: 0.68). Blast count, complex karyotypes, and mutation type or location did not significantly impact OS, highlighting the importance of VAF and platelet count in defining disease aggressiveness under the updated guidelines.
https://doi.org/10.1007/s00277-024-06054-7
Selective menin-KMT2A inhibitor JNJ-75276617 (bleximenib) in KMT2A- and NPM1-altered leukemias
JNJ-75276617 (bleximenib) is a novel, potent, and selective inhibitor that disrupts the interaction between menin and KMT2A, which is crucial for KMT2A- or NPM1-altered leukemias. In preclinical studies, JNJ-75276617 reduced the expression of menin-KMT2A target genes and showed strong antiproliferative activity in AML and ALL cell lines, as well as patient samples, both in vitro and in xenograft models. The compound also exhibited synergistic effects with other leukemia therapies, such as gilteritinib, venetoclax, and azacitidine, and is currently being investigated in clinical trials for relapsed/refractory acute leukemias.
https://doi.org/10.1182/blood.2023022480
Single or Double Induction With 7 + 3 Containing Standard or High-Dose Daunorubicin for Newly Diagnosed AML
This randomized controlled trial evaluated daunorubicin dosing (60 mg/m² vs. 90 mg/m²) and the necessity of a second induction cycle in 864 newly diagnosed AML patients aged 18-65 years. Results showed no significant differences in composite complete remission rates (90% vs. 89%), 3-year relapse-free survival (54% vs. 50%), or overall survival (65% vs. 58%) between the two daunorubicin doses. Among patients with good early response, a second induction cycle offered no significant benefit in relapse-free or overall survival. These findings suggest that 60 mg/m² daunorubicin and single induction are sufficient for this population.
https://doi.org/10.1200/JCO.24.0023
Menin Inhibition With Revumenib for KMT2A-Rearranged Relapsed or Refractory Acute Leukemia
This phase II portion of the AUGMENT-101 trial evaluated revumenib, a menin-KMT2A interaction inhibitor, in patients with relapsed/refractory (R/R) KMT2A-rearranged (KMT2Ar) acute leukemia. Among 94 treated patients (median age 37 years), the most common grade ≥3 adverse events were febrile neutropenia (37.2%), differentiation syndrome (16.0%), and QTc prolongation (13.8%). In 57 efficacy-evaluable patients, the rate of complete remission or CR with partial hematologic recovery (CR + CRh) was 22.8%, significantly exceeding the null hypothesis of 10%. The overall response rate was 63.2%, with 68.2% of responders achieving undetectable residual disease.
https://doi.org/10.1200/JCO.24.00826
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.