howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Acute Promyelocytic Leukemia
Updated on: 28.07.25
Introduction:
- It is a type of acute myeloid leukaemia characterized by presence of abnormal promyelocytes.
- It is almost always associated with PML-RARA fusion gene
- Compared to other AMLs, this has high tendency towards coagulopathy, hemorrhage, and early death
Epidemiology:
- Annual incidence: 0.08 cases / 100 000 population
- 5-8% of AML
- Rarely seen in <10 years, incidence steadily increases during teen years and reaches a plateau during early adulthood
- Common in people with increased body mass index
Etiopathogenesis:
- t (15:17) (q24;q21)
↓
Fusion of retinoic acid receptor α gene on chromosome 17q12 with a nuclear regulatory factor on chromosome 15q22 (promyelocyte leukemia gene)
↓
Formation of PML-RARαfusion gene
↓
PML-RARα fusion gene product enhances interaction of nuclear co-expressor molecule and histone deacetylase
↓
Block in transcription and differentiation of granulocytes.
- Fusion protein also causes disruption of interaction of retinoic acid and RARA
↓
RARα normally acts as transcription activator and inhibits PML protein.
↓
Maturation arrest of hematopoietic precursors at promyelocyte stage
Classification:
- Low risk- TLC- <10,000/cmm (If platelet count is <40,000/cmm, it is classified as intermediate risk)
- High risk- TLC- >10,000/cmm
Clinical Features:
- Haemorrhagic manifestations are prominent- Haemoptysis, haematuria, vaginal bleeding, malena, hematemesis, IC bleed
- Risk of thrombosis if also high
- DIC is common
Investigations:
- Hemogram
- Normocytic normochromic anaemia
- nRBCs are often present
- Sometimes pancytopenia
- Thrombocytopenia
- Increased number of abnormal promyelocytes. They are of 2 types:
- Hypergranular- They have variable nuclear size and shape. Nucleus is usually kidney shaped or bilobed or apple core shaped. Cytoplasm is densely packed with large granules, staining bright pink, red or purple (Sometimes fine dust like). Auer rods are seen in some cells. Faggot’s cells are often seen. These are cells in which bundles of Auer rods are seen. Auer rods in APML are larger than, those seen in other AML
- Microgranular / hypogranular APL- They have apparent paucity/ absence of granules. Auer rods are rarely seen. Predominantly bilobed nuclear shape is noted.
- Bone marrow aspiration and biopsy
- Hypercellular
- Sheets of abnormal promyelocytes present
- Cytochemistry
- MPO – Strongly positive
- NSE – Weakly positive
- Clotting tests:
- PT, APTT- Prolonged.
- Fibrinogen- May be decreased
- Immunophenotyping
- Cells show high side scatter with moderate CD45 positivity (Granulocyte gate).
- Low side scatter is seen in hypogranular variant.
- Positive- CD33, CD13, CD117, MPO
- Often positive- CD64
- Negative- HLA – DR, CD 34 (Microgranular variant may show expression of CD34 and CD2)CD15, CD65, CD11b, CD11c, Cd14, CD16, CD19, CD56, CD3
- Differential diagnosis on flow cytometry:
- Recovering bone marrow with increased promyelocytes: These cells are CD117 negative and positive for CD11b and CD15.
- Acute monoblasticleukaemia- MPO is negative and HLA-DR- Positive
- HLA-DR negative AML
- Cytogenetics
- t(15:17) (q22;q12) (PML/RARA)
- Additional cytogenetic abnormalities are seen in 40% cases. Deletion 7q and trisomy 8 are the most frequent
- Prognostic implication of additional abnormalities is not known.
- PML-RARA testing by RT-PCR/ FISH/ Immunostaining with antiPML monoclonal antibodies on dry smears
- Type of PML-RARA include (depending on the PML breakpoint location on chromosome 15)
- Long (located in intron 6, bcr1)
- Variant (located in exon 6, bcr2)
- Short (located in intron 3, bcr3).
Technique | Advantage | Disadvantage |
Karyotyping | Highly specific | Expensive |
FISH | Less expensive | Fails to fusion signal in presence of non-reciprocal rearrangements |
RT-PCR | High specificity and sensitivity | Poor RNA yield may give false negative results |
Immunostaining | Rapid diagnosis |
|
- NGS panel for myeloid mutations:
- FLT3: Often positive in those patients with high counts. Not prognostically important.
- NRAS: Not prognostically important.
Criteria for Diagnosis:
- Essential
- Myeloid neoplasm with increased peripheral blood and/or bone marrow atypical promyelocytes (may be <20%).
- Detection of PML::RARA.
- No history of exposure to cytotoxic therapy.
- Desirable
- Detection of t(15;17)(q24;q21).
Prognosis:
- Good with high cure rates (>90%)
Pretreatment Work-up and starting of treatment (Both should be done together as soon as diagnosis of APML is suspected):
- History
- Examination
- WHO P. S.
- BSA
- BMA and Bx
- Flow cytometry for acute leukemia panel
- Cytogenetics
- FISH/ PCR- for PML-RARA
- Haemoglobin
- TLC, DLC
- Platelet count
- Peripheral smear
- Coagulation tests: PT: APPT: Fibrinogen:
- LFT: Bili- T/D SGPT: SGOT:
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid
- LDH
- HIV
- HBsAg
- HCV
- UPT
- Prognostic category
- ECHO/ MUGA Scan (If anthracycline planned): LVEF- %
- ECG (If arsenic planned)- QTc:
- PICC line insertion (Once bleeding risk has subsided), Chest X ray after insertion to document tip position
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Tumor board meeting and decision
- In case of suspicion of APML, start Arsenic trioxide +/-ATRA+/- Daunomycin (as per protocol) without waiting for PML-RARA results.
- If low cardiac output, do not give anthracycline. Continue with rest of chemotherapy.
- Give hydration and allopurinol for tumor lysis prophylaxis
- Tab. Prednisolone 0.5mg/Kg- OD (As prophylaxis against differentiation syndrome)
- Cap. Hydroxyurea- SOS
- Attach supportive care drug sheet (No fluconazole)
- Monitoring for initial 2 weeks- Daily/12hrly Screening hemogram, K, PT, APTT, Fibrinogen, creatinine, Uric acid, Calcium, Phosphorous on Mon/Thu- SGPT, Bili, Creatinine, Mg, Once a week- ECG for QTc.
- Daily weight check and Strict I/O monitoring
- Transfuse 4RDPs/ ½ SDP if platelet count is <50,000/cmm
- Transfuse FFP (20ml/kg- Adults- usually 4)- To keep PT and APTT near normal.
- Transfuse Cryoprecipitate (1bag/10kg)- if Fibrinogen is <150mg/dL
- Transfuse PRBC if Hb is <8gm/dL
- Avoid drugs that cause QT prolongation
- Hold Arsenic trioxide if QTc is >500mSec and restart once it is <460mSec, electrolytes replete, syncope and irregular heartbeats cease. (Calculation of QT- Calculate number of small boxes from start of Q wave to start of T wave. 1 small box is equal to 0.04sec. QTc= QT/Square root of RR interval).
- Permanently discontinue arsenic trioxide if there is cardiac arrythmia or severe neurological toxicity.
- Target K+- 4. Give KCl as necessary over 6-10hrs. Target Mg++- 2. Give MgSO4 if necessary
- Strictly no G-CSF to be given during induction. May be considered if there is lifethreating neutropenia during consolidation.
- Avoid routine use of antifibrinolytics and heparin.
- Inform primary care physician
Treatment Plan:
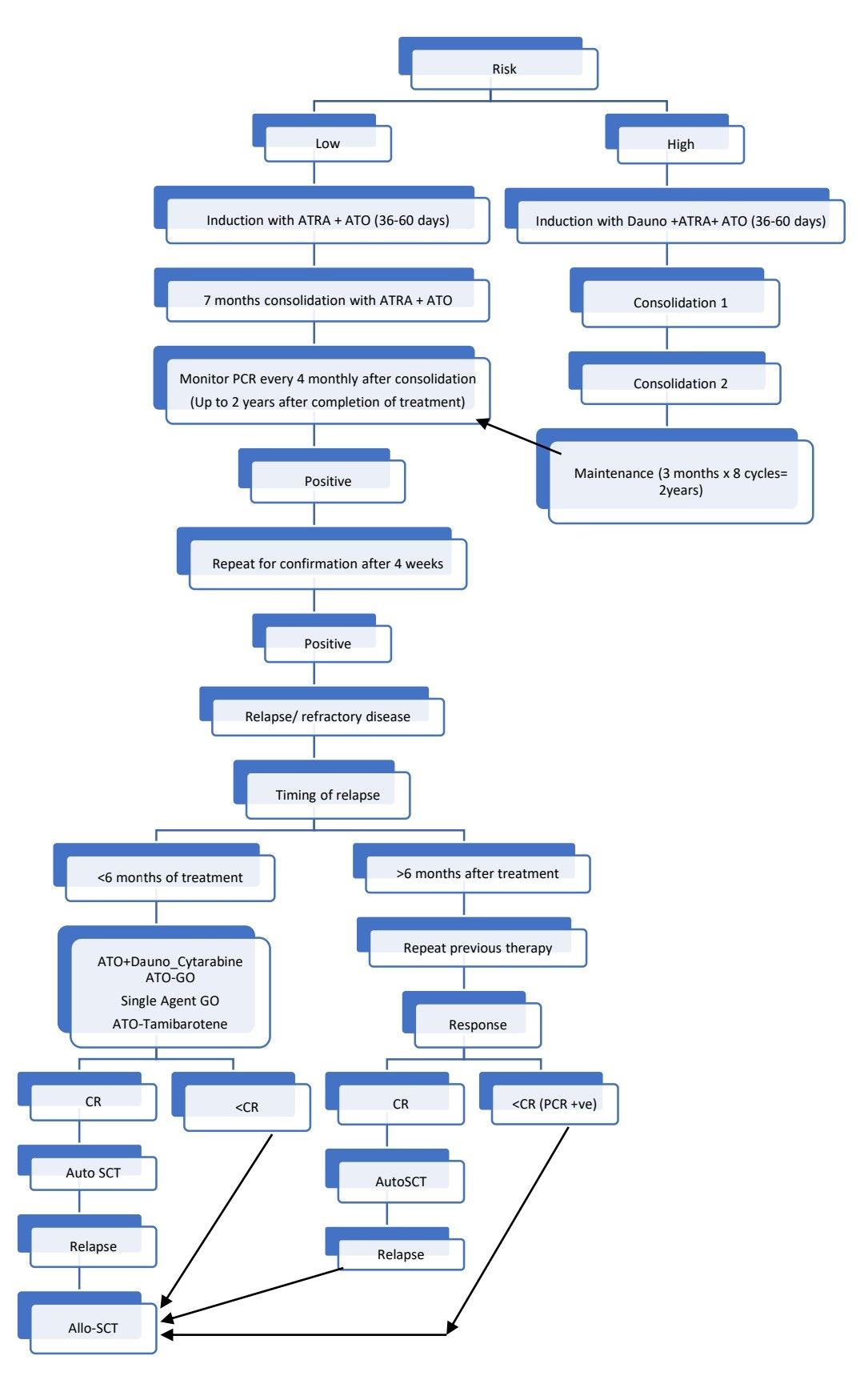
- For children and adolescents:
- ATRA-25mg/m2/day
- Arsenic trioxide- 0.15mg/kg/day
- For differentiation syndrome
- Occurs due to maturation of APML tumour cells into more mature cells and sudden rise in counts after starting treatment. Mediated through proinflammatory cytokines such as IL-1, IL-𝛽, IL-6, IL-8, and tumor necrosis factor ɑ
- Presents with fever, shortness of breath, hypoxia, pleural/ pericardial effusion, tachycardia, and edema.
- Differential diagnoses: heart failure, pneumonia, infection, pulmonary embolus, diffuse alveolar hemorrhage
- Strict I/O monitoring and Daily weight check twice. Also look for edema.
- Prophylaxis- Tab. Prednisolone- 0.5mg/Kg from Day 1. If differentiation syndrome develops in spite of this, Inj. Dexa 10mg- BD for 5 days, then taper over 2 weeks.
- Start Cap. Hydroxyurea
WBC Count in cells/cmm | Dose |
5,000- 10,000 | 500mg, OD |
10,000-15,000 | 500mg, BD |
15,000-20,000 | 500mg, TID |
20,000-50,000 | 500mg, QID |
>50,000 | 1,000mg, QID |
-
- Consider interrupting ATRA and Arsenic trioxide if patient continues to decompensate, exhibiting severe organ dysfunction or requiring admission to the ICU.
- Use anthracyclines if necessary
- Hepatic impairment
- Increase in bilirubin, SGOT/ SGPT >5X ULN, temporarily withold ATRA. If hepatotoxicity persists, temporarily withold arsenic trioxide. Resume ATRA and ATO at 50% of dose once SGOT/SGPT reduces to <4XULN. If no toxicity with this dose, increase the dose of both to 100%.
- Renal impairment
- GFR- <50mL/min- Decrease dose of ATRA to 25mg/m2/day
- GFR- <30ml/L- Give 50% dose of arsenic trioxide
- Pseudotumorcerebri (Severe headache with nausea/ vomiting, visual disorders and papilledema)
- Hold ATRA
- Start dexamethasone
- Opioids for pain
- After disappearance of symptoms, start 50% dose of ATRA and give same dose for 4 days. If no recurrence of symptoms, resume at full dosage.
- Hematological toxicity
- No dose adjustment during induction
- During consolidation, if ANC <1000/cmm or Platelet count <50,000/cmm- Reduce dose of ATRA to 37.5mg/m2 and Arsenic trioxide to 0.11mg/m2.
- ATRA and Arsenic trioxide are contraindicated in pregnancy
- No need for rutine antifungal prophylaxis as this chemo as not very immunosuppressive. There are drug interactions with azoles (Increase levels of ATRA by Cyt P450 inhibition) and amphotericin (cuases potassium wasting) with this protocol.
Protocols used in treatment of APML
High risk (APML4 Protocol, Harry J. Iland)
- Induction
- Inj. Daunorubicin- 60mg/m2 in 100ml NS over 45min- IV- OD- on Day 1 and 2
- Tab. ATRA- 45mg/m2 PO, in 2-3 divided doses. Days 1 to 36
- Inj. Arsenic trioxide 10mg in 250ml NS over 2hrs- Days 9 to 36 (26days)
- Continue ATRA and Arsenic trioxide till CR is achieved or maximum up to 60 days
- BM biopsy: at end of induction
- Consolidation- 2 cycles. Start 3-4 weeks after the end of induction
- Tab. ATRA- 45mg/m2 PO, in 2-3 divided doses. Days 1 to 28
- Inj. Arsenic trioxide 10mg in 250ml NS over 2hrs- Days 1to 28
- Inj. Methotrexate- 12.5mg IT- On days- 1 and 15
- Maintenance(Start 3-4 weeks after the end of consolidation cycle 2)
- 8 cycles of 3 months each (total 2 years)
- ATRA (45mg/m2 PO, in 2-3 divided doses) to be administered alone for the first 2 weeks of each cycle
- 6 mercaptopurine (50-90mg/m2- PO-OD) and Methotrexate (5-15mg/m2- PO- ONCE A WEEK) to be given for the remainder of each cycle, targeting a neutrophil count of 1-2 x 109/L with dose adjustments for excessive myelosuppression or hepatotoxicity.
- Follow up once a month with CBC, SGPT, Bili and Creatinine.
Low risk (APL0406 Intergroup Study AL WP GIMEMA – DSIL protocol):
- Induction:
- Tab. ATRA- 45mg/m2 PO, in 2-3 divided doses. Days 1 to 36
- Inj. Arsenic trioxide 10mg in 250ml NS over 2hrs- Days 1 to 36
- Continue ATRA and Arsenic trioxide till CR is achieved or maximum up to 60 days
- BM aspiration and biopsy: at end of induction
- Consolidation: (Start 3-4 weeks after the end of induction)
- Arsenic trioxide (10mg in 250ml NS over 2hrs) 4 weeks on 4 weeks off, for a total of 4 courses
- ATRA (45mg/m2 PO, in 2-3 divided doses) 2 weeks on and 2 weeks off for a total of 7 courses
- No maintenance for low-risk patients
(If Arsenic trioxide is not available or contraindicated, Idarubicin or gemtuzumab ozogamicin containing regimens may be used)
Response Criteria:
- Complete remission:
- No clinical evidence of APML
- ANC >1500/cmm
- Unsupported platelet count- >1lac/cmm
- Bone marrow- Normocellular to moderately hypocellular with <5% abnormal promyelocytes/blasts
- If there is doubt about achievement of CR, BM must be repeated after interval of 2-3 weeks while keeping patient on ATRA and in meanwhile refrain from new therapeutic interventions.
- Molecular remission assessment (which is done by PCR) should be done after consolidation.After induction, it is usually positive, which does not indicate resistant disease.
- Usually
- Leukemic promyelocytes disappear from peripheral blood by 2-4 weeks
- Bone marrow becomes normal by 4-10 weeks
- PCR becomes negative after second consolidation
About Each Modality of Treatment:
- ATRA
- Mechanism of action
- ?Induces synthesis of protein that selectively degrades PML-RARA
- Overcomes the recruitment of histone deacetylase activity by PML-RARA fusion gene through interference with a nuclear corepressor.
- Induction and activation of activator of transcription factor STAT-1.
- Mechanism of action
- Arsenic trioxide
- Mechanism of action: Induces apoptosis of abnormal promyelocytes by increasing H2O2 levels and NF kappa beta inhibition.
Other Treatment Options:
- Single agent arsenic trioxide:
- Adults- 10mg in 250ml NS, Children- 0.15mg/kg in 200ml NS over 2-3 hours
- Low risk:
- Induction- for 42 days or until CR (Maximum up to 60 days)
- 4 weeks interval
- Consolidation 4 weeks
- 4 weeks interval
- Maintenance: 10 days a month for 6 months
- High risk
- Induction- for 42 days or until CR (Maximum up to 60 days)
- 4 weeks interval
- 1st Consolidation 4 weeks
- 4 weeks interval
- 2nd Consolidation 4 weeks
- 4 weeks interval
- Maintenance: 10 days a month for 12 months
Monitoring After Treatment/ Follow-up:
- PCR every 3 months for 2 years after remission induction.
- Risk of relapse is extremely low, 3 years after achieving CR1.
Special Situations:
- Therapy related APML
- Treated similar to de-novo APML
- APML in elderly
- Rare
- Treatment is similar to standard protocol
- APML in children
- ATRA is given in a dose of 25mg/m2/day, as there is higher risk of pseudotumorcerebri. These symptoms disappear with diagnostic LP. If these symptoms persist, discontinue ATRA for some time. Same time add acetazolamide and dexamethasone.
- APML in pregnant
- Avoid retinoids in first trimester, as they are teratogenic.
- Arsenic is embryotoxic, hence it must be avoided during entire pregnancy.
Related Disorders:
- APML with Variant translocations
- t(11:17) (q23:q21)
- RARA on chromosome 17 fuses with promyelocytic leukemia zinc finger (PLZF) on chromosome 11
- Usually, ATRA and ATO resistant
- Auer rods are absent
- Nucleus - Regular; condensed chromatin pattern; Pelger like cells
- Cytoplasm - Granularity; midway between classic M3 and FAB M2
- CD56 expression is often present
- t(5;17) (q23;q12)
- Nucleophosmin gene on chromosome 5 (NPM1) fuses with RARA
- Sensitive to ATRA therapy
- t(11:17)(q13;q22)
- Nuclear matrix associated (NuMA) gene on chr.11 fuse with RARA
- Isochromosome 17
- Other fusion partners of RARA include: STAT5B at 17q21, PRKAR1A at 17q24, FIP1L1 at 4q12, BCOR at Xp11, OBFC2A at 2q32, TBLR1 at 3q26, GTF2I at 7q11, IRF2BP2 at 1q42, and FNDC3B at 3q26.
- t(11:17) (q23:q21)
Figures:
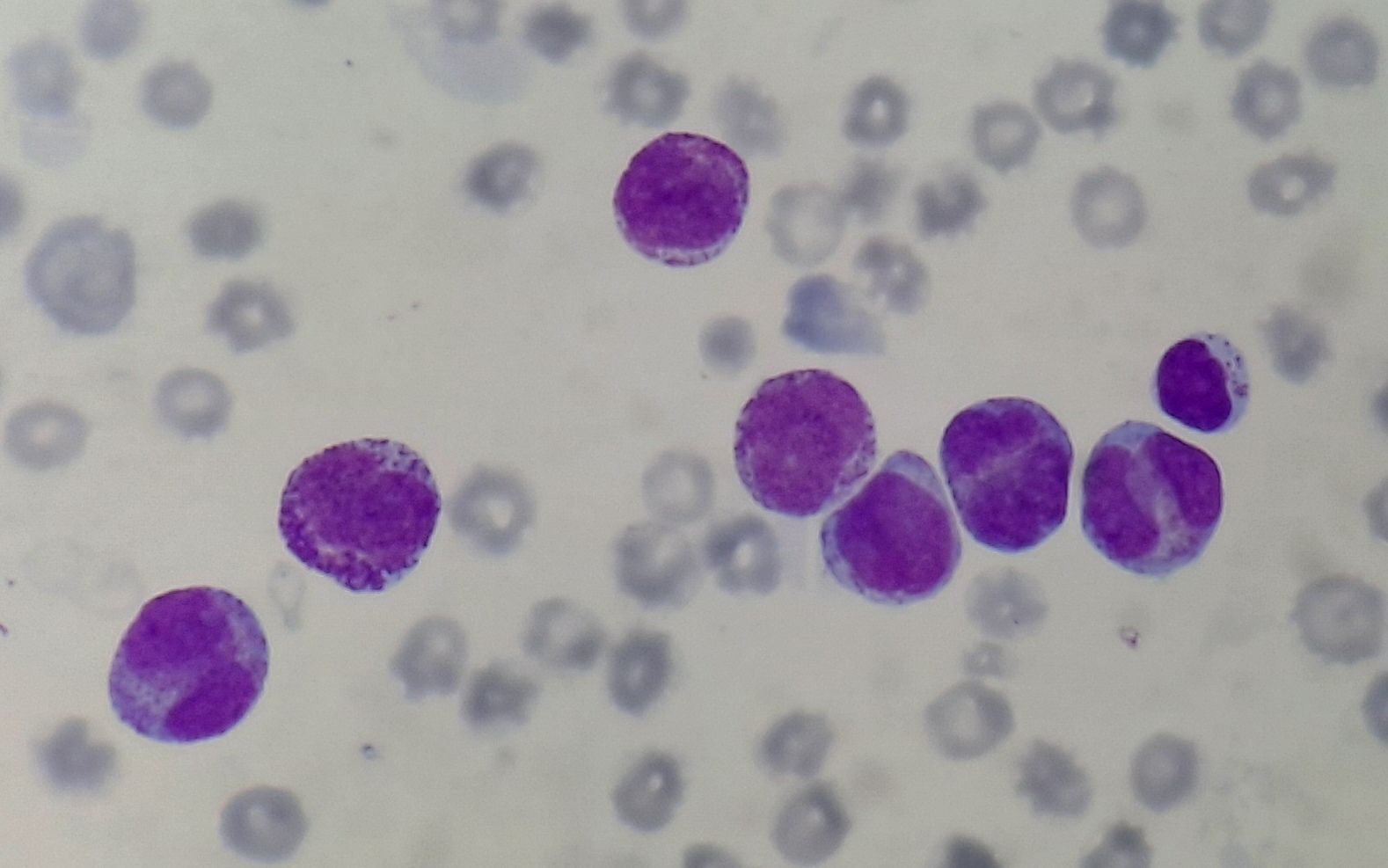
Figure 4.3.1- Acute promyelocytic leukemia- Peripheral smear
Recent advances:
Autologous hematopoietic cell transplantation during second or subsequent complete remission of acute promyelocytic leukemia
A recent study analyzed 296 patients with APL who had undergone autologous HCT during second or subsequent complete remission (CR2+) between 2006 and 2019. The 5-year probabilities of relapse-free survival, overall survival, relapse, and nonrelapse mortality for the entire cohort were 85%, 88%, 9%, and 6%, respectively. The study showed very favorable long-term results when autologous HCT is conducted during CR2 + across the various subgroups of patients with relapsed APL.
https://doi.org/10.1038/s41409-021-01501-9
Venetoclax for arsenic-resistant acute promyelocytic leukaemia
Some patients with APML experience relapse due to arsenic resistance with or without PML mutations. Venetoclax suppresses oxidative phosphorylation and selectively target leukaemic stem cells.In this pilot study venetoclax was added CAG regimen (cytarabine, aclarubin and granulocyte colony-stimulating factor) (V-CAG) for the treatment of arsenic-resistant relapsed and refractory APL. Total of nine patients were enrolled. Eight patients achieved complete remission, among whom three achieved complete molecular remission, and one achieved partial remission.
https://doi.org/10.1111/bjh.18061
Arsenic trioxide and all-trans retinoic acid without chemotherapy in acute promyelocytic leukaemia
This study evaluated the outcomes of treating acute promyelocytic leukemia (APL) patients of all risk classes using arsenic trioxide (ATO) and all-trans retinoic acid (ATRA) without chemotherapy. The treatment approach involved induction and consolidation therapy with ATO and ATRA, followed by maintenance therapy for intermediate- and high-risk patients. Out of 206 patients, the majority were intermediate risk (51.9%) followed by high risk (43.2%). The estimated 5-year event-free survival (EFS) and overall survival (OS) rates for the entire cohort were 79% and 80%, respectively. T
https://doi.org/10.1111/bjh.18618
Risk factors of thrombosis in Chinese subjects with acute promyelocytic leukemia
Thrombosis is a common complication in patients with acute promyelocytic leukemia (APL). A study involving 44 consecutively Chinese APL patients investigated risk factors for thrombosis. One arterial and six venous thrombotic events occurred in the patient cohort. The study found that PAI-1 gene 4G4G type, CD15, WT-1, and FLT3-ITD mutations were associated with an increased risk of thrombotic events in Chinese APL patients. These findings can help in identifying patients at higher risk of thrombosis and guide preventive measures and management.
https://doi.org/10.1186/s12959-021-00294-7
Arsenic-induced neurotoxicity in patients with acute promyelocytic leukaemia
A large multicenter retrospective study in Australia evaluated 487 acute promyelocytic leukemia (APL) patients treated with arsenic-based therapy from 2008 to 2023 to investigate the incidence of neurotoxicity and its association with obesity and cumulative arsenic dose. Neurotoxicity occurred in 23% of patients, mainly as peripheral neuropathy, with the majority being grade 1-2 in severity. Incidence of neurotoxicity increased with BMI: 16% in non-obese, 25% in obesity class I, and 41% in obesity class II-III. Obesity, weight >100 kg, daily arsenic trioxide dose >15 mg, and cumulative induction dose >500 mg were significantly associated with neurotoxicity.
https://doi.org/10.1111/bjh.19297
A complete oral regimen for induction therapy of patients with high-risk APL
An all-oral regimen of all-trans retinoic acid (ATRA), oral arsenic (realgar–indigo naturalis formula, RIF), and oral etoposide (VP16, median dose 1000 mg) was effective and safe for high-risk acute promyelocytic leukemia (APL). Complete hematologic remission (CHR) and complete molecular remission (CMR) rates were comparable to those achieved with intravenous chemotherapy. The 2-year overall survival was 100% in the oral VP16 group. This regimen provided a convenient alternative during the COVID-19 pandemic.
https://doi.org/10.1111/bjh.19464
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.