howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Beta Thalassemia
Introduction:
- Thalassemia is a heterogenous group of inherited anemiascharacterized by defects in synthesis of one or more globin chain subunits of hemoglobin tetramers.
- Thalassic stands for sea, as most of the patients were of Mediterranean origin.
Epidemiology:
- Common in Mediterranean, middle east, India, Myanmar regions (areas where malaria was/is endemic, as thalassemia gives protection against malaria)
- Beta thalassemia major births in India- 8,000-10,000/ year
- Common in Sindhi community
- Total thalassemia transplants in India- Nearly 150/year
Classification:
- Classification is phenotypic based (not genotypic based and not based on hemoglobin electrophoresis) which depends on clinical severity and transfusion requirement.
- Thalassemia major- Require regular blood transfusions (at least 8/ year) to maintain hemoglobin above 6gm/dL. HbA- Absent and HbF is 92-95%
- Thalassemia intermedia- Hemoglobin is usually above 6gm/dL without transfusions. They require blood transfusions occasionally, especially during periods of stress such as pregnancy. They are at risk of non-transfusional iron overload. They may require splenectomy. HbA- 10-30% and HbF is 70-90%. Refer to thalassemia intermedia section for details.
- Thalassemia minor- Completely asymptomatic. They can have slightly low hemoglobin or hemoglobin level is often normal. MCV and MCH are low with slight erythrocytosis. HbA2 level is increased (3.5-7%) and HbF is 1-3%.
Etiology:
- It is an autosomal disorder with co-dominant/ autosomal recessive inheritance. Hence heterozygotes are usually asymptomatic.
- More than 200 mutations have been described that result in partial to complete absence of β-gene expression.
- Homozygotes and compound heterozygotes usually suffer from thalassemia syndrome.
Clinical Types and their genotypes:
β- Thalassemia type | Genotype (See below) |
Thalassemia major (Cooley’s anemia/ Mediterranean anemia) | β0 /β0 or β+ /β+ |
Thalassemia intermedia | β0 /β or β+ /β+ |
Thalassemia minor/ Trait | β0 /β or β+ /β0 |
- β0 -Total absence of beta chain synthesis
- β+ -Decreased chain synthesis
- β -Normal Chain production (Either normal gene or mutated gene with normal beta chain synthesis)
Mutations Causing β thalassemia | |||
Phenotype | Number of Mutations described | ||
Transcriptional mutants | |||
Promoter | Silent Mild β+ | 2 5 12 | |
5 UTR | Silent Mild β+ | 4 1 1 | |
RNA processing | |||
Splice junction | β0 | 21 | |
Consensus splice sites | Silent β0 Mild β+ | 1 1 1 8 | |
Cryptic splice sites in introns | β0 /β+ β0 β+ | 1 1 3 | |
Cryptic splice sites in exons | Mild β+ | 2 3 | |
3’-UTR RNA cleavage; poly (A) signal | Mild β+ | 4 2 | |
RNA translation | |||
Initial codon | β0 | 7 | |
Nonsense codon | β0 | 14 | |
Frame shift | β0 | 64 | |
Deletions | β0 | 17 | |
Dominant thalassemias | |||
Missense mutations | β0 | 8 | |
Deletion or insertion of intact codon | β0 | 7 | |
Nonsense mutations | β0 | 2 | |
Frameshift or aberrant splicing | β0 | 14 | |
Common beta thalassemia mutations in India
- IVS- 1-5 (G→C)- 48%
- 619 bp deletion- 18%
- IVS-1 (G→T)- 9%
- Frameshift 41/42 (-CTTT)- 9%
- Frameshift 8/9 (+G)- 5%
- Codon 15 (G→A)- 5%
- IVS II-837 (T→G)
So deficiency of β- chain is because of
- Promoter gene mutation- Because of this, RNA polymerase fails to bind to DNA, so there is no transcription.
- Chain termination mutation – This mutation induces formation of a nonsense codon
- Splicing mutation – In this case splicing of mRNA does not occur and all unspliced RNA is destroyed within nucleus.
Deficiency of β chain leads to
- Lack of HbA formation leading to microcytic hypochromic anemia
- Aggregation and precipitation of free α chains in red cell precursors leading to cell membrane damage and ineffective erythropoiesis
- Hemolytic anemia as alpha inclusions interfere with passage of RBCs through splenic sinusoids
Causes for increased HbF
- Cells producing beta chain die while those producing gamma chain survive and proliferate.
- Severe anemia leads to stressed erythropoiesis. Because of this there is switching of production of HbF from HbA.
Pathogenesis:
1.
Anemia
↓
Hypoxia
↓
Increased erythropoietin
↓
Expansion of marrow all over the body
↓
Deformities of skull and face
Porosity of long bones
Extramedullary hematopoietic tumors
Splenomegaly
- Diversion of nutrients to ineffective RBC precursors leads to poor development and wasting
- Massive turnover of erythroid precursors leads to hyperuricemia (gout) and folate deficiency.
- Constant exposure of spleen to RBC inclusions leads to splenomegaly which in turn worsens anemia due to increased RBC removal and expansion of plasma volume.
- Hypoxia causes increased intestinal absorption of iron. Frequent transfusions lead to transfusionalsiderosis. Both together lead to accumulation of iron in Kuppfer cells of liver and macrophages in spleen and also within endocrine glands and heart. Finally this leads to hemochromatosis.
Clinical Features: (Severity depends on amounts of HbA and HbF produced)
- Anemia- Insidious in onset, starts at around 4-6 months, when synthesis of gamma chain i.e. HbF starts decreasing
- Jaundice
- Failure to thrive and loss of body fat
- Anorexia, diarrhea
- Irritability
- Recurrent fever- Due to hypermetabolic state and recurrent infections.
- Facial deformities
- Flattened nose, wide set eyes, bossing of skull, prominent malar eminence and hypertrophy of upper maxillae (Mongoloid facies)
- These changes occur due to striking expansion of red bone marrow which leads to thinning of cortical bone of maxilla & frontal bone (“Chipmunk” facies and crew hair cut appearance on X-ray)
- Cardiac problems due to iron overload
- Reversible myocyte failure
- Arrythmias including heart block
- Pulmonary hypertension
- Thrombotic stroke due to atrial fibrillation
- Cardiac failure due to constant high cardiac output- It is the most common cause of death in untreated children
- Liver problems
- Infectious hepatitis due to HBV and HCV
- Cirrhosis of liver due to iron deposition
- Hepatocellular carcinoma
- Growth retardation- Seen in 25-28% patients. Causes include:
- Transfusional iron overload
- Chelation toxicity
- Nutritional deficiency- especially vitamin D
- Growth hormone deficiency
- Chronic liver disease
- Hypogonadism
- Hypothyroidism
- Psychosocial stress
- Multiple endocrine abnormalities
- Hypogonadism
- Hypothyroidism
- Impaired glucose tolerance and diabetes mellitus
- Osteoporosis: Seen in 40-50% cases. Causes include
- Marrow expansion
- Hypogonadism, hypoparathyroidism and hypothyroidism
- Iron deposition in osteoid
- Vitamin D deficiency
- Decreased physical activity
- Splenomegaly and hepatomegaly may be noted due to
- Reticulo endothelial cell hyperplasia
- Extramedullary hematopoiesis
- Iron deposition
- Increased proneness to infections due to
- Increased levels of iron favors bacterial growth especially Yersiniaenterocolitica
- Blockage of macrophage-monocyte system due to increased rate of dextruction of RBCs
- Increased turnover of red cell precursors leading to hyperuricemia& secondary gout
- Hypersplenism – Leading to thrombocytopenia and bleeding tendency
- Deformities of skull
- Poorly formed teeth & malocclusion
- Inadequate drainage of sinuses & middle ear leading to chronic sinusitis & deafness
- Tumors composed of extra-medullary hematopoiesis
- Thrombosis- Especially after splenectomy
- Chronic leg ulcerations
Investigations:
- Hemogram:
- Hemoglobin content- 3-4gm/dL (When beta thalassemia major child becomes symptomatic)
- MCV- Reduced to <67fL
- MCH – Decreased
- MCHC – Normal/decreased
- RDW – Less than 18 in beta thalassemia trait(In iron deficiency anemia it is >19)
- Microcytic hypochromic RBCs
- Severe anisocytosis and poikilocytosis with presence of schistocytes, ovalocytes, dacrocytes and target cells,
- Polychromatophilic cells and nucleated RBCs are seen
- Neutrophilia with shift to left
- Platelets are usually normal
- Bone marrow examination
- Cellularity is increased
- Erythroid hyperplasia with M:E ratio of 0.1 or less
- Increased basophilic and polychromatic normoblasts
- Cytoplasm of normoblasts is scanty and it contains scattered siderotic granules
- Foamy cells similar to Goucher’s cell may be noted (Foam is a result of partially digested red cell membrane lipids associated with intense ineffective erythropoiesis)
- Reticulocyte count – Increased but <10%
- Methyl violet staining: Granular cytoplasmic inclusions can be demonstrated which represent aggregates of α chains
- Hb electrophoresis: Elevated Hb A-2 and HbF levels
- Osmotic fragility test: Increased resistance to hemolysis. Complete hemolysis is not seen even in distilled water.
- Urine examination: It is dark brown in color due to presence of dipyrrole breakdown products of hemoglobin
- Serum bilirubin – Elevated
- Serum haptoglobulin –Absent
- 51 Cr labeled RBC survival study – Shortened survival
- Molecular techniques to demonstrate specific genetic mutations
- PCR for commonly known mutations
- Sequencing to identify new mutations
- S. Ferritin levels
- Correlates with body iron stores
- Relatively easy and inexpensive test
- Decreasing trend indicates decreasing iron load. Increasing trend may occur even with decreasing iron load. It can be due to inflammatory state as well.
Prognosis:
- Untreated patients survive for less than 4 years
- Sporadically transfused children survive for 10-20 years and die because of CCF.
- Those who have adequate transfusions have normal development, but present with problems of iron overload at the end of first decade.
- Adequate transfusion and chelation is associated with longevity and good quality of life.
Pretreatment Work-up: Do following things prior to first transfusion.
- Hemoglobin HPLC
- Complete RBC antigen profile or at least typing of C, c, D, E, e and Kell. As later on differentiating autoantibodies from allo antibodies is difficult.
- HIV, HBsAg and HCV testing
- LFT, RFT, Ferritin
- Hepatitis B vaccination and start all age-appropriate routine immunizations.
Routine monitoring during treatment:
- Every visit- History, examination (Height, Weight, Liver and spleen size), CBC
- Once in 4 months- LFT, Creatinine, Ferritin (start after 10 unit transfusions) and adjust the dose of chelation therapy.
- Yearly once
- MRI to assess liver and cardiac iron overload
- Endocrine evaluation (starting from 6 years)- Appropriate treatment of any abnormalities
- Bone density by Dexa scan (starting from 2 years)
- HIV, HBsAg, HCV testing
- Thyroid function test, Vitamin D levels, Fasting glucose
- ECG and 2D Echo
- Audiogram and ophthalmology check up if on Desferal
Treatment:
- Transfusion therapy
- Start transfusions only when hemoglobin falls to less than 6gm/dL and make sure other factors such as febrile illness/ folate deficiency are not confounding the assessment of severity of anemia. If this precaution is not taken, a thalassemia intermedia child may be wrongly labeled as thalassemia major, which ends up with chronic transfusion therapy followed by long-term chelation therapy. Identifying the nature of disease at a later date will be extremely difficult. Genotype may provide some guidance in distinguishing major from intermedia, but still clinical assessment is most important in distinguishing these two entities.
- Anemia alone is not an indication of need for chronic transfusions. Some children with hemoglobin level of >6gm/dL, may benefit from chronic transfusion therapy, if they have following features.
- Poor growth and development
- Development of bone changes
- Signs of increased cardiac effort
- Tachycardia
- Sweating
- Administer 10-20ml/Kg over 4-6hrs (with mid-transfusion frusemide), once in 2-5 weeks to maintain pretransfusion hemoglobin of above 9gm/dL. Interval is optimized based on pretransfusion hemoglobin values during every visit.
- Transfuse ABO and Rh (C, c, D, E, e) and Kell compatible blood.
- Use leucoreduced RBCs (Prestorage filtration/ Pretransfusion filtration/ bedside filters)
- Avoid use of first degree relatives as blood donors, to decrease problems during BMT and also to prevent t-GVHD.
- Use washed PRBCs in patients who develop >2 non-hemolytic transfusion reactions
- Uses of transfusions include
- Improvement of anemia
- Suppression of compensatory mechanisms which cause distressing symptoms
- In case of development of alloantibodies, eliminate donors with corresponding antigens. Not all antibodies are clinically significant and may not be able to destroy apparently incompatible red cells at body temperature. Alloimmunization rate is approximately 16%. If transfusion is very difficult, use steroids/ IVIg/Rituximab.
- Folic acid
- Dose- 1mg- OD
- Given to meet increased demand of hypercellular marrow.
- Iron chelation therapy
- Given when ferritin level is more than 1000ng/ml which usually occurs after 10 transfusions or at age of 2 years.
- Ideally Ferritin levels must be maintained below 1000ng/ml. But levels below 2500ng/ml will also have beneficial effect.
- Ferritin level must be measured once in 4 months.
- Liver iron estimation is most reliable indicator of iron overload. But, it is not practical to do.
- Other methods include superconducting quantum interference device (SQUID) and MRI with special software for iron estimation. They are also very expensive and are not easily available.
- Chelation at correct doses and frequency can balance iron excretion with iron accumulation.
- Aim should be to prevent iron accumulation, rather than removing accumulated iron, as damage caused is usually irreversible.
- Drugs used for chelation are
- Deferoxamine
- Dose: 30-60mg/Kg – SC/IV infusion over 8-10 hours- for 5 days a week
- Vitamin C- 22-3mg/kg should be given at the time of infusion, as it increases availability of chelatable iron
- 10mg hydrocortisone may be added to infusion mixture to decrease local reactions
- Not easily available and expensive
- Excellent safety and efficacy profile
- Can cause retinal defects, cataract, sensory neural hearing loss, local reactions
- Deferasirox (Exjade, Asunra, DefriJet- Available as 250mg and 500mg tablets)
- Now most commonly used chelator world-wide
- Dose- 20-40mg/kg- OD- Disperse tablet in water/in apple juice using a non-metallic stirrer. It should be given 30min prior to meals
- Side effects include:
- Diarrhea, vomiting, and abdominal pain. No need to decrease the dose in such situations. Give the tablet along with food
- Increased creatinine and liver enzymes- Both are harmless
- Skin rashes- Stop if severe and restart at lower doses.
- Chelation is as effective as deferoxamine
- Can be given to children as young as 2 years
- Adjust the dose to maintain ferritin between 500-1000ng/ml
- Use with caution in patients with renal/hepatic impairment
- Deferiprone (Ferriprox, Kelfer- Available as 250 and 500mg tablets)
- Dose: 25mg/kg- TID
- Side effects include
- Gastrointestinal symptoms
- Arthralgia- Can be controlled with NSAIDs
- Agranulocytosis- Seen in 1.7% patients
- Red colored urine- due to excreted iron
- Efficacy is same as deferoxamine
- Adjust the dose with ferritin level (But should not exceed daily dose of 100mg/kg)
- Monitor ANC initially weekly, then along with each transfusion.
- Combination therapy
- Not approved so far
- Used only if monotherapy proves to be ineffective
- Deferoxamine
- Splenectomy
- Should have a guarded approach and it should be done only if absolutely necessary. Splenomegaly due to periods of under-perfusion may be reversible. Hence prior to deciding about splenectomy an adequate transfusion program for several months should be followed and then re-evaluated.
- Associated with high incidence of cerebral thrombosis, venous thromboembolism and pulmonary hypertension.
- Should be performed after 5 years of age (Splenectomy before 5 years often leads to life threatening infections)
- Indications:
- Dramatic increase in transfusion requirement (>200ml/kg/year of PRBC)
- Pain due to increasing splenomegaly
- Hypersplenism with severe cytopenias
- Massive splenomegaly with possibility of rupture
- Splenectomy is sufficient for thalassemia intermedia
- It decreases transfusion requirement by 30% and decreases annual iron overload by 40%.
- If patient has cholelithiasis, during same surgery, cholecystectomy should be performed.
- Prior vaccination and postspenectomy penicillin prophylaxis has to be given.
- Bone marrow transplantation from HLA identical sibling
- It is the only treatment with possibility of cure.
- Now, standard of care if there is HLA identical sibling.
- Early referral to transplant center is recommended
- BMT should be carried out in 1st few years of life before there is iron overload, so that success rate is about 90%
- Avoid doing transplant after 7 years of age, as it is associated with poor outcome.
- Considering lifelong blood transfusion and chelation and management of complications, BMT is certainly a cost effective option if adequate expertise exists, even in developing countries.
- Pretransplant evaluation to note adequacy of iron chelation (developed by Pesaro group) includes 3 factors:
- Ferritin>1000ng/ml
- Liver fibrosis in liver biopsy
- Liver >2cm below costal margin.
Class | Number of factors | Disease free survival at 5 years after BMT |
I | 0 | 90-93% |
II | 1 or 2 | 86% |
III | 3 | 62% |
- Prior to transplant, hypertransfusion (to suppress BM) and intense chelation are practiced at some centers.
- BM harvest should be used as source of stem cells
- MUD/Haplo/Cord transplants are associated with poor outcome- Better to do them in an experimental set up.
- Myeoablative conditioning is preferred over RIC, as with latter there is increased risk of recurrence of disease. Better results observed with treosulphan containing regimens.
- Mixed chimerism is seen in 10% of patients. 30% of them eventually reject the graft. Even with 20% donor engraftment, one can achieve normal hemoglobin levels.
- In adults, overall survival is 65% and transplant related mortality is 35%.
Dietary advice
- Avoid red meat
- Drink black tea with meals to decrease iron absorption
- Avoid vitamin C with meals
- Diet rich in calcium including milk, cheese and oily fish are recommended
- Juice from leaf buds of wheat grass- Increases intervals between transfusions
- Avoid alcohol as it enhances liver problems.
- Calcium and Vitamin D Supplementation
Other Treatment Options:
- Hydroxyurea
- Increases HbF level
- Dose- 5-10mg/Kg
- Monitor counts every 3-4 weeks
- Every 8 weeks increase the dose by 2.5-5mg/kg/day, making sure that ANC is >2000/cmm
- 40% patients have modest increase in hemoglobin level
- Better effects in thalassemia intermedia
- Adding Erythropietin/ Darbapoietin results in better hemoglobin improvement
- Thalidomide
- Dose- 25-100mg/day
- Leads to rapid increase in total hemoglobin and HbF
- Hypomethylatingagenys- 5Azacytidine/Decitabine- No more used in view of toxicity
- Sotatercept
- JAK2 Inhibitors
- Gene therapy
- Done using somatic cells that are reprogrammed to induce pleuripotent stem cells
- Lentiviral vector-TNS 9 is used to induce normal gene.
- Reimplantation is done by autologous stem cell transplant after myeloablative/ RIC conditioning.
- Possible complication- Insertional mutagenesis. But this may not happen, as leukemia development needs mutation of several other genes and late erythroids in which gene transfer is done are not capable of producing leukemia.
Prevention:
- Screening the total populations while the children still are in school and warning carriers about potential risk of marriage to another carrier.
- Prenatal diagnosis to be done if both are carriers.
- Antenatal diagnosis
- Analysis of fetal DNA obtained from amniotic fluid (late pregnancy) / chorionic villi biopsy (can be done by 9 weeks of gestation. It is better, as adequate DNS can be easily extracted)
- PCR is done for same mutations which are found in parents.
Related Disorders:
- δβ- Thalassemia
- Complete absence of both β and δ chain synthesis
- 100% Hemoglobin is HbF
- Unlike hereditary persistence of fetal hemoglobin, increased γ chain synthesis fails to fully compensate for β chain production
- They have mild anemia (Hemoglobin-10-12g/dL)
- Peripheral smear - Microcytic hypochromic picture
- Fetal hemoglobin is heterogenously distributed in RBCs, which differrentiates it from HPFH.
- γδβ- Thalassemia
- Occurs due to deletion / inactivation of entire β chain complex
- Homozygous state is incompatible with life
- Heterozygous patients have severe hemolytic anemia in newborns and mild anemia in adulthood.
- Hemoglobin Lepore
- Clinical features similar to thalassemia
- Non α chain is a δβglobin hybrid
- N-terminal end of δ chain is fused to C terminal end of β chain
- Etiology- Aberrant recombination of misaligned δ & β genes during meiosis
- Hb lepore is functionally normal but as δβ chain synthesis is under the control of δ promoter gene (which limits synthesis of δ chain gene to 2.5% that of β chain ) hybrid globin chain are ineffectively synthesized
↓
Excess of α chains
↓
Precipitation of α chains
↓
Premature destruction of RBCs
(In effective erythropoiesis)
- Homozygous Hb lepore
- Clinical features- Severe anemia, Hepatosplenomegaly, and skeletal abnormalities
- Hemoglobin – 4-11g/dL
- Peripheral smear – Microcytic, hypochromic cells, anisocytosis, poikilocytosis, target cells and basophilic stippling
- Hemoglobin electrophoresis
- No HbA or HbA2
- 8-30% - HbLepore (migrates with HbS in alkaline pH)
- Remaining – Hb F
- Treatment
- Same as beta thalassemia major
- Heterozygous H Lepore
- Asymptomatic
- Hemoglobin- 12-14g/dL
- Hereditory persistence of fetal hemoglobin
- It is a group of heterogeneous disorders in which the absence of δ & β gene synthesis is compensated for by increased γ chain synthesis into adult life.
- Hb A & HbA2 are absent
- Only HbF is present
- No clinical symptoms
- 2 sub types
- Homozygous HPFH- 100% HbF
- Heterozygous HPFH- HbF – 10-30%
- HbE/βthal
- Seen in south east Asians
- Can present as thalassemia.
- Clinical manifestation depends on whether patient has β+ or β-
- Mild: Hemoglobin- 9-12 gm%, Skeletal changes may be present
- Moderate- Hemoglobin- 6-7gm%, Presents as thalassemia intermedia
- Severe- Hemoglobin- 4-5gm%, Present with anemia, leg ulcers, bone deformities, marked tendency to infections, iron overload, splenomegaly and extramedullary erythropoietic tumor.
Recent advances:
BetibeglogeneAutotemcel Gene Therapy for Non–β0/β0 Genotype β-Thalassemia
In a recent study by Locatelli et al gene therapy for transfusion depended beta thalassemia was evaluated. After myeloablation with busulfan, patients were given beti-cel intravenously. Transfusion independence was observed in 91% patients with average haemoglobin levels of 11.7gm/dL. Safety profile was same as busulfan based myeloablation.
DOI:10.1056/NEJMoa2113206
Figures:
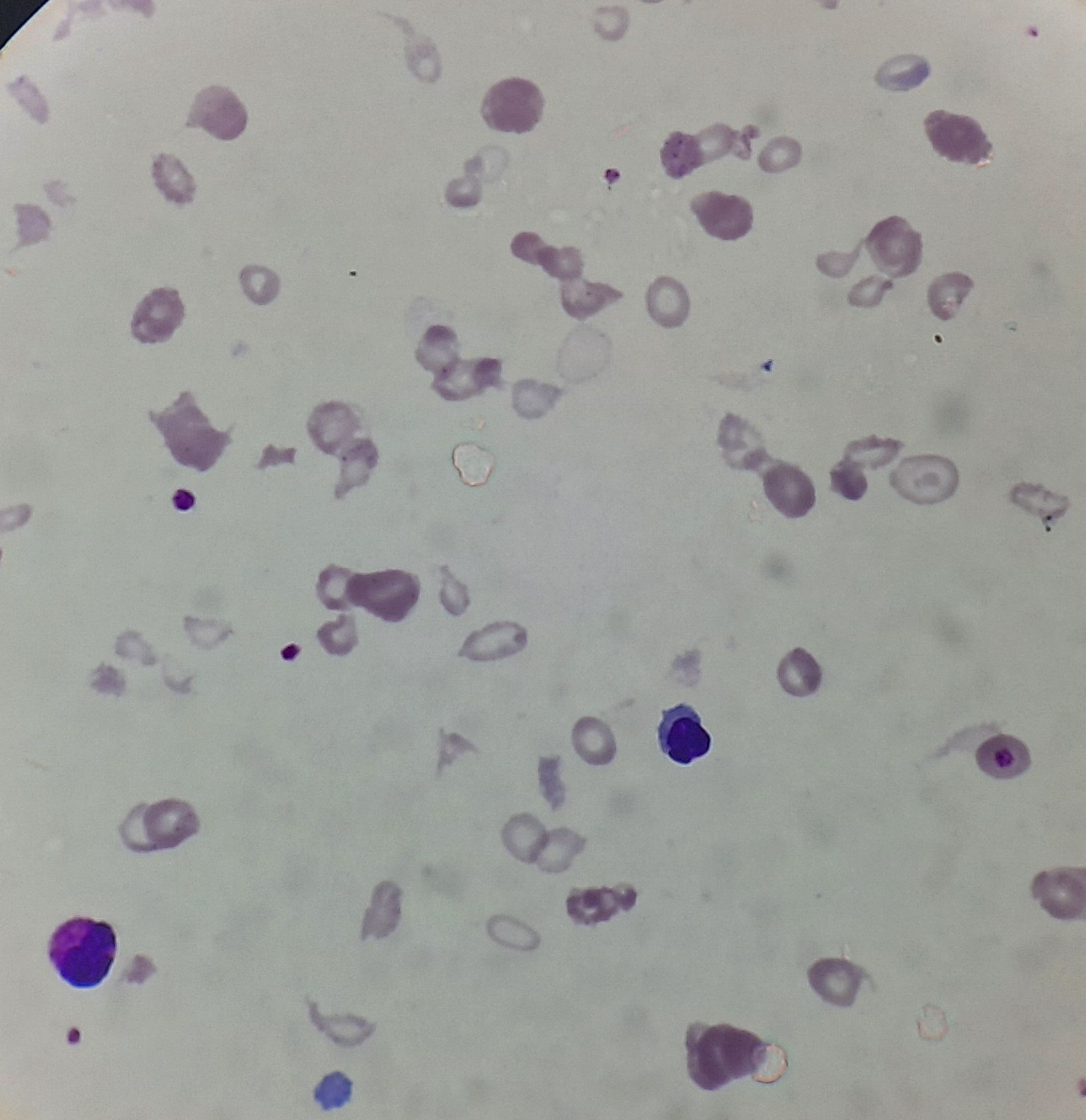
Figure 8.12.1- Beta thalassemia major- Peripheral smear
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.