howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Burkitt Lymphoma
Introduction:
- It is a highly aggressive B cell lymphoma charectorised by the translocation and deregulation of c-myc gene on chromosome 8.
- If bone marrow contains more than 25% tumor cells, then the condition is called as Burkitt leukemia
- Accounts for 30-50% of pediatric lymphomas
Pathogenesis:
- Genetic aberrations during immunoglobulin rearrangements or attempted immunoglobulin class switching in B cell precursor
- Juxtaposing MYC to the IGH locus by t(8;14)à Translocation between MYC and an immunoglobulin promoter à Constitutive expression of MYC
- Other translocations include: t(8;22) and t(2;8)
Classification:
- Endemic
- Commonly involves jaws and facial bones
- Seen in equatorial Africa
- EBV associated
- Seen at 4-7 years
- Sporadic
- Usually presents as abdominal mass in ileocecal region
- Low EBV association
- Median age- 30 years
- Immunodeficiency associated BL – Seen in HIV patients
Clinical Features:
- Endemic – Loosening of teeth and extrusion of eye – occurs due to mandibular and maxillary bone involvement.
- Sporadic – Bulky mass per abdomen – Due to involvement of kidney, adrenals, ovary and lymph nodes.
Investigations:
- Biopsy of lesion/ lymph node mass
- Diffuse monotonous pattern of infiltration of medium sized tumour cells.
- Nuclei are round with clumped chromatin and relatively clear parachromatin. Multiple basophilic centrally located nucleoli are seen. Cytoplasm is deeply basophilic and contains lipid vacuoles
- High mitotic count
- Multiple apoptotic bodies
- “Starry sky pattern” – Imparted by numerous benign macrophages that have ingested apoptotic tumor cells
- Immunophenotype
- Positive – Membrane IgM with light chain restriction, CD45, B cell associated Antigens(CD19, 20, 22, 79a, PAX 5), CD10, TCL1, BCL6, CD38, Cd77, CD21, BCL6
- Ki67 (High growth fraction >95%)
- Negative- CD5, CD3, CD23, TdT, BCL1, BCL2, MUM1, CD44, CD138, CD25, CD30
- Molecular studies
- Clonal rearrangements of immunoglobulin heavy a light chain genes
- Somatic mutations of Ig genes are found
- Cytogenetics/ FISH
- t(8:14)- Translocation of MYC on 8q24 to immunoglobulin heavy chain region on 14q32.
- cMYC expression by FISH is required for confirmation of diagnosis
- Less common – t (2:8), t (8:22)
Staging by Murphy &Hustu
Stage | Definition |
I | A single tumor (extranodal) or single anatomic area (nodal) with the exclusion of mediastinum or abdomen |
II | A single tumor (extranodal) with regional node involvement. Two or more nodal areas on the same side of the diaphragm Two single (extranodal) Tumors with or without regional node involvement on the same side of the diaphragm. Primary gastrointestinal tract Tumor, usually in the ileocoecal area, with or without involvement of associated mesenteric nodes only. |
IIR | Completely resected abdominal disease |
III | Two single Tumors (extranodal) on opposite sides of the diaphragm. Two or more nodal areas above and below the diaphragm. All primary intrathoracic Tumors (mediastinal, pleural, thymic) All paraspinal or epidural Tumors, regardless of other tumor site (s). All extensive primary intra-abdominal disease. |
IIIA | Localized but unresectable abdominal disease. |
IIIB | Widespread multiorgan abdominal disease. |
IV | Any of the above with initial CNS and /or bone marrow involvement |
Prognosis:
- Highly aggressive but potentially curable (up to 90%)
- Patients without relapse for 2 years can be considered as cured
- Poor prognostic markers:
- Higher stage
- BM or CNS involvement
- Unresected tumor measuring >10cm in diameter
- High LDH
Pretreatment Work-up:
- History
- B-Symptoms
- Examination
- LN:
- Spleen:
- WHO P. S.
- BSA
- IHC/Flow cytometry
- BMA and Bx
- CT (CAP)
- Stage
- Hemoglobin
- TLC, DLC
- Platelet count
- LFT- Bili- T/D SGPT: SGOT: Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid:
- LDH
- HIV:
- HBsAg:
- HCV:
- LP- CSF- IT MTX
- UPT
- Cytogenetics
- FISH- t (8;14)
- Risk category
- ECHO (If anthracyclines planned) LVEF- %
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
Treatment Plan:
Paediatric Burkitt: Refer to R-LMB Intergroup B NHL 2010 trial protocol in NHL Section. Click here
Risk stratification:
Low risk: Both criteria must be met
- Normal LDH
- Stage 1 and completely resected abdominal mass
Or Single extra-abdominal mass <10cm
High risk:
- Stage I with mass >10cm
- Stage II to IV
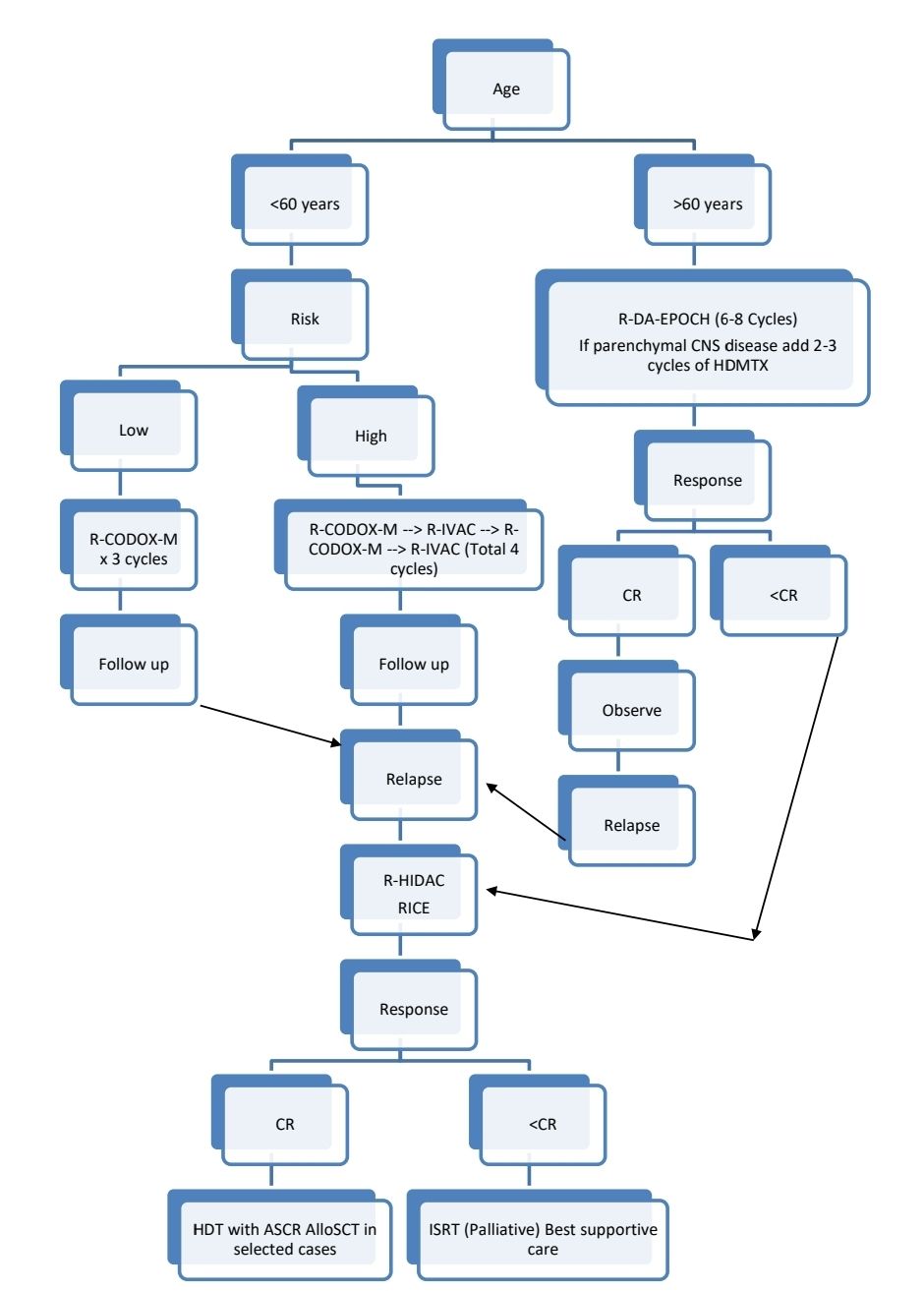
Monitoring After Treatment/ Follow-up:
- History, examination and labs- every 2-3 months for 1 year, then 3 monthly for 1 year and then every 6 monthly
- CT (C/A/P)- Only if clinically indicated
Figures:
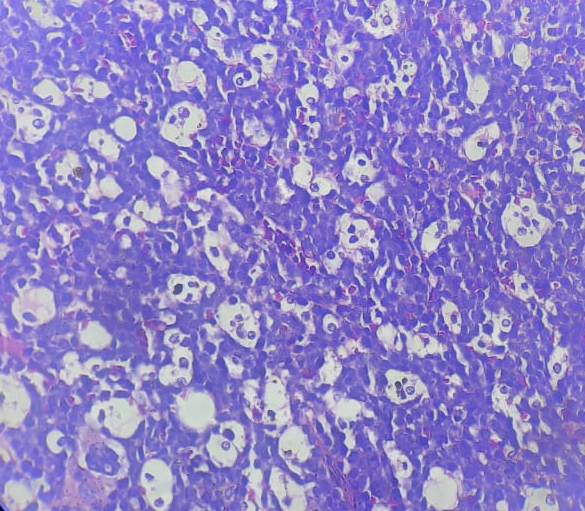
Figure 6.16.1- Burkitt leukemia/ lymphoma- Bone marrow biopsy
Recent advances:
R-CODOX-M/R-IVAC versus DA-EPOCH-R in patients with newly diagnosed Burkitt lymphoma
This multicentre, phase 3, open-label, randomised study compared two treatment regimens for newly diagnosed high-risk Burkitt lymphoma: R-CODOX-M/R-IVAC and DA-EPOCH-R. The study closed prematurely due to slow accrual. Of the 89 enrolled patients, 84 were included in the modified intention-to-treat analysis. The 2-year progression-free survival was 76% in the R-CODOX-M/R-IVAC group and 70% in the DA-EPOCH-R group. While DA-EPOCH-R did not demonstrate superior progression-free survival, it was associated with fewer toxic effects and supportive care requirements. The study suggests that DA-EPOCH-R is a valid therapeutic option for high-risk Burkitt lymphoma without CNS involvement.
https://doi.org/10.1016/S2352-3026(23)00279-X
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.