howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Chronic Myeloid Leukemia
Updated on 20.03.2025
Introduction:
- It is a myeloproliferative disease that originates in an abnormal pleuripotent bone marrow stem cell and is consistently associated with Philadelphia chromosome and/ or BCR/ABL fusion gene
Epidemiology:
- It is the most common MPN
- Comprises of 15-20% of all leukemias
- Incidence- 2 cases/ 1 lac population
- Most common in 5th to 6th decade (peak age 40-49 years). Lower median patient age in low income countries.
- Has slight male predominance
- Accounts for 2% of all leukemias in children aged <15 years
Etiology: Not known in most of the cases
- Exposure to high dose irradiation. Ex-In treatment of ankylosing spondylitis
- Toxic chemicals
- Viral agents
Pathogenesis:
As a part of random chromosomal break in case of radiation exposure etc., there can be break causing reciprocal translocation of long arm of chromosome 22 to chromosome 9 – t (9;22) (q34;q11)- (Philadelphia chromosome)
Resulting chromosomes are labeled as
del 22q – (Philadelphia chromosome)- Minus because part of long arm is missing
der9q+ - Plus because the long arm is longer than the normal
↓
Movement of segment of Abselon proto-oncogene (ABL) from long arm of chromosome 9 to major breakpoint cluster region (M-BCR) on chromosome 22
↓
Fusion of 5’ BCR and 3’ ABL genes in a head to tail fashion in derivative 9q + chromosome
↓
The fusion BCR/ABL gene is under the control of promoter region of BCR gene and transcribes an 8.5 kb chimeric mRNA
↓
8.5 Kb chimeric mRNA produces a specific onco-protein of 2,10,000 Daltons (p210) which has abnormally high tyrosine kinase activity
↓
As tyrosine kinase receptors serve as receptors for growth factors and promote growth, cells with high tyrosine kinase activity have a proliferating advantage
(This involves JAK/STAT, PI3/AKT and RAS/MEK pathways)
↓
Over a period of years, the t (9;22) cell lines replace the normal bone marrow and CML is expressed
Types of BCR-ABL fusion genes:
- Types of breakpoints in BCR gene
- Downstream exon 13 (e13 type), exon 14 (e14 type)
- Downstream exon 1 (e1)
- Types of breakpoints in ABL-1 gene
- Upstream of exon-2 (ABL-1 a2 fusion type)- Most common
- Downstream exon 2 (ABL-1 a3 fusion type)- Rare
- So common fusion m-RNAs are
- e13a2 and e14a2
- Found in 98% of CML
- This results in formation of p210 oncoprotein
- These mRNAs are named- Major BCR-ABL1 fusion subtype
- e1a2
- Found in 75% of Ph+ve ALL and <1% CML
- This results in formation of p190 oncoprotein
- This mRNA is named- minor BCR-ABL1 fusion subtype
- Other fusions- e6a2,e8a2, e19a2 etc
- Found in 2-3% of CML
- Fusion of ABL with μBCR results in formation of p230 protein. This causes complete neutrophilic maturation in CML
- e13a2 and e14a2
- Hence type of BCR ABL transcript must be determined prior to treatment, so that monitoring can be done later. By this, false negative tests can be safely excluded later.
- BCR-ABL genes can be found in normal individuals in very low levels. As there are no additional changes which are necessary to produce leukemia, these individuals do not develop CML.
- In 5-10% of cases of CML (according to FAB classification) the Ph chromosome is absent. In 50% of these cases, the translocation is masked at karyotyping level, but molecular BCR/BCR fusion can still be detected by FISH or PCR
- 2-5% of childhood ALL and 25% of adult ALL have Philadelphia chromosome
- Disease progression usually occurs because of additional chromosomal abnormalities and additional mutations. 75% cases of accelerated phase/ blast crisis are accompanied by development of new chromosomal abnormalities (clonal evolution). They include
- Additional Ph chromosome
- 3q26.2 rearrangement*
- Trisomy 8
- Isochromosome 17q*
- Loss of Y chromosome
- Trisomy 19
- Trisomy 21
- Monosomy 7*
- Complex karyotypes*
- Rarely- Trisomy 9, t (15; 17), t (3:21), t (3:3) or inv (3)
- Mutation of p53, N-ras, K- ras, RUNX1, RB1, myc, p16, CDKN2A, AML1, EVI1, TET2, CBL, ASXL1, IDH1 and IDH2
(*- Additional chromosomal anomalies associated with increased risk of progression of disease)
Clinical Features:
- Chronic Phase (Lasts for 2-7 years)
- Asymptomatic- 20-40% (Found to have high blood counts on routine evaluation)
- Vague non-specific symptoms, abdominal discomfort, early satiety
- Anemia – Tiredness, breathlessness
- Weight loss, lethargy, anorexia, sweating
- Splenomegaly- Moderate, firm, smooth and painless
- Hepatomegaly
- Rarely bone and joint pain – Tenderness over lower sternum
- Bleeding tendency: due to platelet dysfunction
- Rarely- Gout, priapism, tinnitus, stupor due to hyperleucocytosis, abdominal pain due to splenic infarct, vasopressin reactive diabetes insipidus, acne urticaria associated with hyperhistaminemia, visual disturbances due to retinal hemorrhage, acute febrile neutropenic dermatosis (Sweet's syndrome) and digital necrosis
- Accelerated phase / Blast crisis (seen 30-40 months after diagnosis of CML)
- It is transformation of CML into AML or ALL (70% are AML and 20% are ALL, most commonly ALL-L2)
- Increasing fatigue, fever, night sweats, weight loss
- Bleeding
- Bone tenderness
- Marked Pallor
- Appearance of lymphadenopathy
- Increase in splenomegaly
- Blast crisis leads to death within 1-2 months of onset
- Rarely chronic phase may progress to myelofibrosis or osteomyelosclerosis (overgrowth of bony trabeculae in marrow)
Investigations:
- Hemogram
- Normocytic normochromic / hypochromic anemia
- nRBCs may be seen
- Leucocytosis (> 100 x 109 / L)
- Excess of granulocytic cells, shift to left in myeloid cells with predominance of myelocytes and neutrophils.
- Myelocyte bulge/ Leukemic hiatus- Exaggerated proportion of myelocytes compared to proportion observed in normal persons
- Signs of myeloid dysplasia including Pelger –Huet anomaly & decreased leukocyte alkaline phosphatase
- Blast count is usually < 2% (more than 20% indicates blast crisis)
- Eosinophilia
- Basophilia – Consistent feature in CML. Marked increase indicates impending blast crisis
- Thrombocytosis (seen in 50% patients)
- Occasionally megakaryocytes and micro megakaryocytes may be seen
- Thrombocytopenia is seen in blast crisis
- Bone marrow aspiration
- Required at diagnosis, since
- Proportion of blast cells and basophils is important to identify advanced phases of disease.
- BM is better sample for cytogenetics
- Hypercellular with left shifted myelopoiesis (75-90% cellularity)
- Myeloid to erythroid ratio increased to 10 : 1 to 50 : 1
- Blast count is usually 2 – 10% (>20% indicates blast crisis)
- Megakaryocytes- Increased in number, immature and atypical forms are seen. Compared to other MPN in CML they are small and hypolobated (Dwarf megakaryocytes)
- 30% of cases show pseudogoucher cells and sea blue histiocytes
- Required at diagnosis, since
- Trephine biopsy
- Thinning of cortex and erosion of trabeculae
- Paratrabecular cuff of immature neutrophils is thickened to 5-10 cells (Normal 2-3 cells)
- Abundant segmented neutrophils are situated deeper in the intertrabecular regions of bone marrow
- Erythroid precursors are reduced in percentage and show normoblastic erythropoiesis
- Increase in reticulin fibrosis. It correlates with increased number of megakaryocytes, larger spleen size and more severe anemia
- Nests of blasts can be noted in biopsy.
- Cytochemistry
- Neutrophil alkaline phosphatase (LAP score) – Decreased (This is helpful in differentiating CML from myeloid leukemoid reaction and PV)
- Immunophenotyping
- Chronic phase: Dysmyelopoiesis (Decreased side scatter, with upregulation of CD56. Rarely aberrant expression of CD10, CD11c, CD16, HLA-DR noted).
- Blast crisis: Flow cytometry is useful in identifying type of blast crisis AML/ ALL.
- Cytogenetics:
- For Ph chromosome and additional chromosomal abnormalities
- Labor intensive
- Sensitivity limit of detection of about 5% Ph-positive cells in a population of normal cells
- Additional chromosomal abnormalities are seen in 15% patients. Common abnormalities include: trisomy 8, isochromosome 17, additional loss of material from 22q or double Ph
- FISH for BCR-ABL
- Done if PCR and cytogenetics are negative
- Useful in identifying unusual variant rearrangements
- More specific compared to cytogenetics
- Can identify unusual variant rearrangements that are outside the regions amplified by the RT-PCR primers.
- Reverse transcription-PCR
- Employs specific primers to amplify a DNA fragment from BCR-ABL1 mRNA transcripts
- Type of transcript (p190, p210 and p239) can be noted as primers for each of them are different.
- 2-4% patients harbor atypical BCR-ABL transcripts lacking exon2
- Use of nested primers and sequential PCR makes this technique very sensitive, capable of detecting 1 abnormal cell in 105-106 normal cells. This helps in follow up evaluation.
- Low cost, sensitive, rapid and not labour intensive.
- Data can be quantitated by including a competitive standard RNA in the reaction.
- Quantification may differ based on internal standards used and the method of obtaining results.
- IS- It is a reference standard which most of labs use to report RT-PCR reports.
- Serum vitamin B-12 levels – Elevated to 10 times the normal (As neutrophils contain transcobalamin I and III)
- Serum uric acid level –elevated
- Serum LDH level – elevated
- Histamine levels- Elevated (It correlates with basophil count)
- S. Potassium- Spuriously elevated due to release from WBCs during clotting of blood in vacutainer
- Levels of angiogenic factors (VEGF, Beta FGF, hepatocyte growth factor)- Elevated
- Platelet function study- Deficiency of secondary wave of aggregation to epinephrine (Due to deficiency of adenine nucleotides in the storage pool)
Criteria for Diagnosis
CML Chronic Phase:
- Essential:
- Peripheral blood leukocytosis (Usually >12,000/cmm)
- Detection of Ph chromosome and/or BCR::ABL1 rearrangement
- Does not meet any diagnostic criteria for blast crisis
- Desirable:
- Bone marrow aspiration and biopsy to confirm disease phase
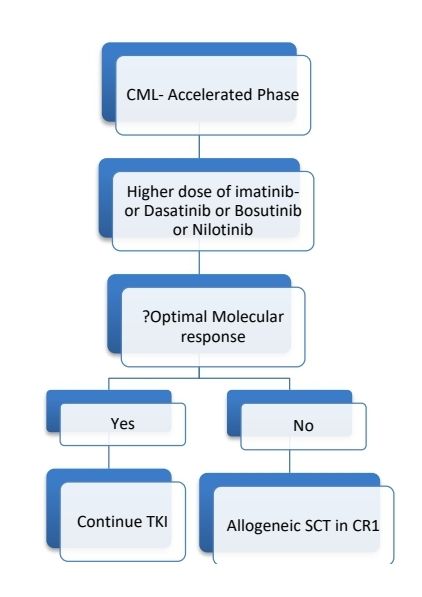
CML Accelerated phase:
- 10–19% blasts in the peripheral blood and/or bone marrow
- ≥20% basophils in the peripheral blood
As per new WHO classification:
With use TKIs, patients in accelerated phase are found to have almost similar survival compared to chronic phase. Hence CML-AP is no more used. However, the WHO identifies the following features as being associated with an increased risk of disease progression.
- At diagnosis
- High ELTS score
- 10–19% blasts in the peripheral blood and/or bone marrow
- ≥20% basophils in the peripheral blood
- Additional chromosomal abnormalities: 3q26.2 rearrangements, monosomy 7, isochromosome 17q and complex karyotype
- Clusters of small megakaryocytes associated with significant fibrosis
- Emerging on treatment
- Failure to achieve a complete haematological response to the first TKI
- Any haematological, cytogenetic, or molecular indications of resistance to two sequential TKIs (excluding explicable causes such as the presence of a kinase domain mutation resistant to the previous choice of TKI)
- Development of new additional chromosomal abnormalities
- Occurrence of compound mutations in the BCR-ABL1 fusion gene during TKI therapy
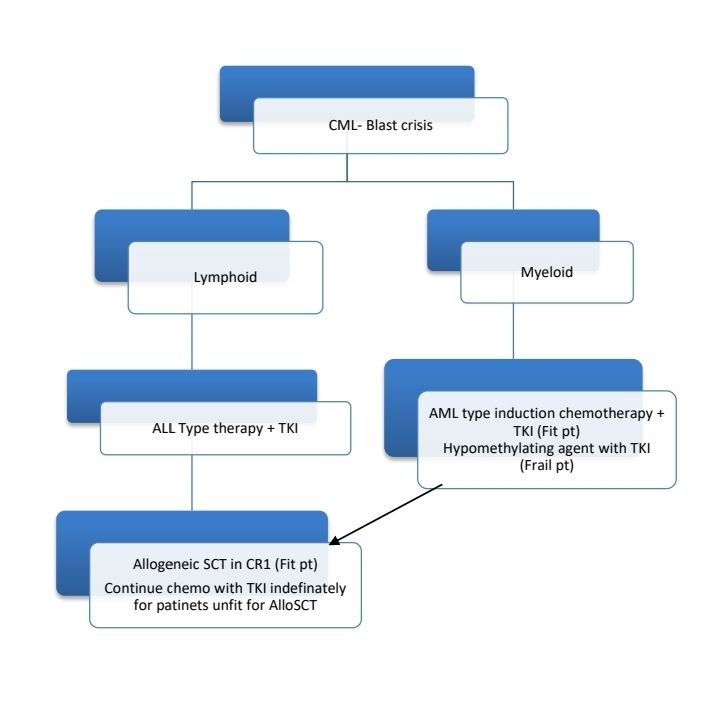
CML-Blast crisis
- Blast ≥20% of peripheral blood white cells or of nucleated bone marrow cells
- Extramedullary blast proliferation – skin, LN, spleen, bone or CNS
- Presence of bonafide lymphoblasts in the peripheral blood or bone marrow (even if <10%)
Prognosis:
- Most important prognostic marker is response to treatment with TKI
- ELTS score: 0.0025 × (age/10)3 + 0.0615× spleen size + 0.1052× peripheral blood blasts + 0.4104× (platelet count/1000)–0.5
- Low-risk: < 1.5680
- Intermediate-risk: 1.5680- 2.2185
- High-risk: > 2.2185
- Click here for online calculator: https://www.leukemia-net.org/leukemias/cml/elts_score/
- Sokal Index, Hosford or EUTOS score: These were other prognostic scoring systems used previously
- Poor prognostic factors are
- Percentage of circulating blasts - > 3%
- Spleen size- >10 cm below costal margin
- Platelet count- > 7,00,000 / µl
- Cytogenetic clonal evolution
- Age- > 60 years
- Leukocyte doubling time
- Poor response to initial treatment
- 9q abnormalities
- Rapid rate of shortening of telomeres
- In general: With Imatinib, 80% patients have progression free survival at 5 years. Most of them live a normal life span.
- Disease progressing to advanced phase while on treatment has worse prognosis compared to de novo advanced phase
- If untreated, average time for progression of disease is 3-5 years.
Differential Diagnosis:
- Leukemoid reaction: Compared to CML it has following features
- Blasts and promyelocytes in peripheral blood are rare
- Neutrophils have toxic granulation, Dohle bodies and cytoplasmic vacuoles
- No absolute basophilia or eosinophilia
- Platelets are usually normal
- Anemia may be present but NRBC not usually seen
- LAP score is increased
- Philadelphia chromosome is absent
- Juvenile myelomonocytic leukemia
- Atypical CML
- Chronic eosinophilic leukemia
- Chronic neutrophilic leukemia
- Other MPNs
Pretreatment Work-up:
- History
- Examination: Spleen (cm below costal margin)
- WHO P. S.
- BMA and Bx
- Hemoglobin
- TLC, DLC
- Platelet count
- LFT: Bili- T/D SGPT: SGOT: Albumin: Globulin:
- Creatinine Uric acid
- Electrolytes: Na: K: Ca: Mg: PO4:
- LDH
- HIV
- HBsAg
- HCV
- UPT
- Cytogenetics
- ECG (For QTc)
- Cardiovascular risk assessment: (www.qrisk.org).
- Quantitative RT- PCR- BCR- ABL1 (Using IS scale)
- Dual fusion FISH for BCR-ABL (If PCR is negative)
- ELTS Prognostic score
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Tumor board meeting and decision
- Inform primary care physician
Treatment Plan:
Pending BCR-ABL studies start hydroxyurea to lower high TLC
During pregnancy, treat with interferons
CML- Chronic Phase
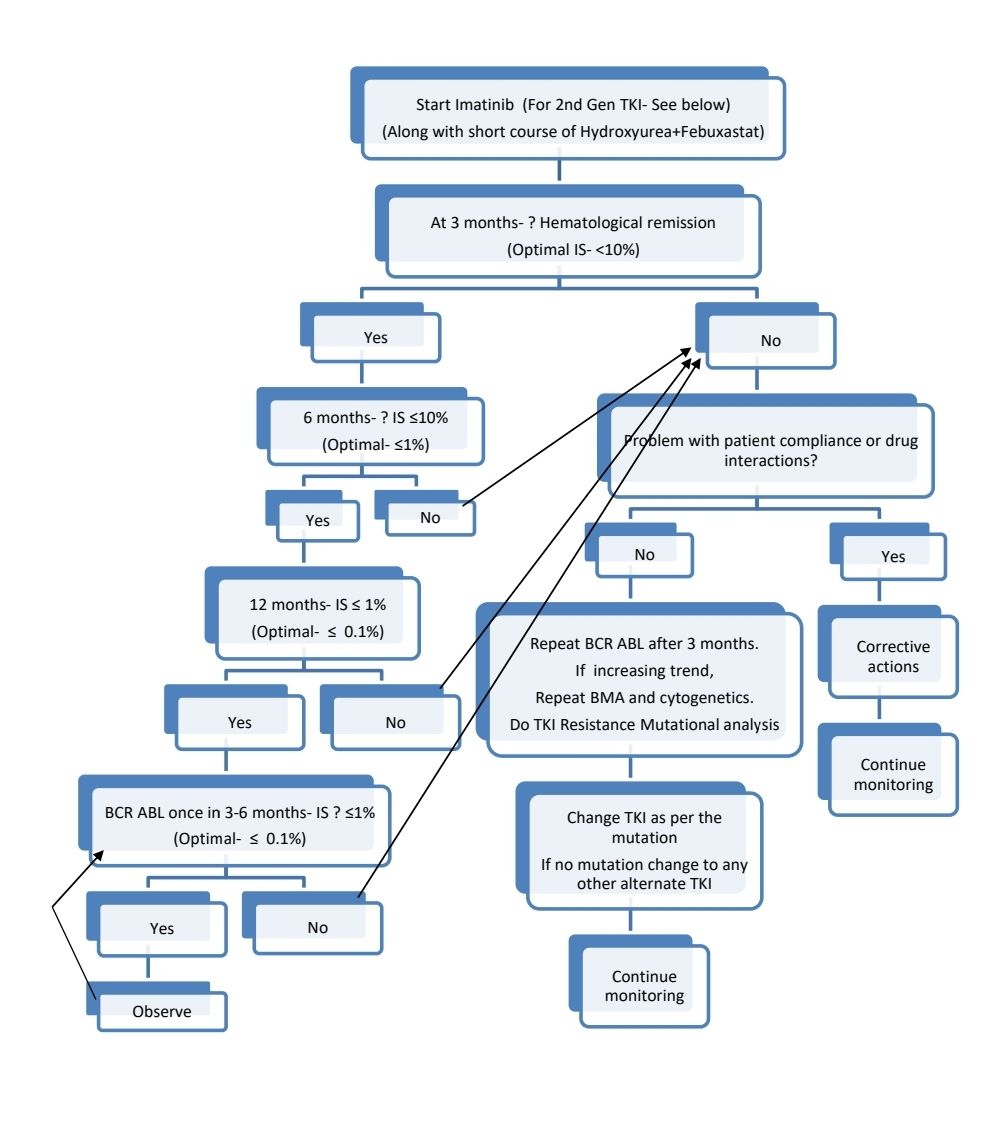
2nd generation TKI or Allosteric TKI (Asciminib) may be started upfront for:
- Patients with intermediate and high Sokal score (although data is not very convincing)
- Patients with additional chromosomal abnormalities at diagnosis
- Woman who wish to have children where rapid molecular response is desirable, so that treatment discontinuation becomes feasible.
- Younger patients with high-risk disease and in whom the aim of therapy is to induce a treatment-free remission status
2nd generation TKIs produce significantly deeper and faster responses but do not have any impact on survival prolongation.
If 2nd generation TKI is started upfront in elderly patients, care must be taken to avoid exacerbation of present comorbidities or inducing new problems with 2nd generation TKIs.
Changing TKI as per the mutation detected:
- F317L/V/I/C, T315A, V299L- Nilotinib, Bosutinib
- V299L- Nilotinib
- Y253H, E255V/K, F359V/I/C- Dasatinib, Bosutinib
- G250E- Dasatinib
- T315I- Ponatinib, Asciminib, Omacetaxine, Allogeneic SCT, Olverembatinib
- No Mutation- Randomly change to any second line TKI
Response Criteria:
- Complete hematological response:
- Complete normalization of peripheral blood counts with TLC <10,000/cmm
- Platelet count <4.5lac/cmm
- No immature cells such as myelocytes/ promyelocytes/ blasts in peripheral blood and <5% basophils on differential count.
- No signs or symptoms of disease with resolution of palpable splenomegaly
- Molecular response (RT-quantitative PCR):
- Should be done on peripheral blood and samples must be processed within 72 hrs. If delay is inevitable, extract RNA, convert to cDNA, which can be stored for longer time. Patients with atypical BCR-ABL1 variants should be monitored using specifically designed primers. Control genes commonly used are: ABL1 and BCR.
- International Scale (IS)- Ratio of BCR-ABL1 transcripts to ABL1 transcripts. It was developed to harmonize molecular responses across laboratories.
- Early molecular response: IS- <10% at 3 months of therapy.
- Major molecular response-BCR-ABL1- >3 log reduction or IS- ≤ 0.1%
- Deep molecular response:
- MR4: >4 log reduction or IS- ≤ 0.01%
- MR4.5: >4.5 log reduction or IS- ≤ 0.0032%
- MR5: >5 log reduction or IS- ≤ 0.001%
- Complete molecular response- Variably described (Usually ≤ 0.0032%). Levels less than assay's level of sensitivity (Ex: >4.5 log reduction)
- Survival rates are similar in patients with transcript levels <1% and those with deeper molecular remissions.
- Average gap between PCR relapse and morphological relapse is 16 months.
- Bone marrow aspirate, cytogenetic analysis and FISH are not required to monitor response. However, previously following response categories were used:
- Complete cytogenetic response: No Ph chromosome positive metaphases
- Partial cytogenetic response: 1% to 35% Ph Positive metaphases
- Major cytogenetic response: Includes both complete and partial cytogenetic response
- Minor cytogenetic response: More than 35% Ph Positive metaphases
About Each Modality of Treatment:
- Tyrosine kinase inhibitors:
- Block the interaction between the BCR::ABL1 oncoprotein and ATP (via competitive inhibition at the ATP-binding site of the BCR::ABL1 oncoprotein), which stops proliferation of malignant cells.
- Adverse effects common to all TKI- Rash, nausea, edema, fatigue, myalgias, arthralgias
- 1st generation TKI: Imatinib
- 2nd generation TKIs: Dasatinib, Nilotinib, Bosutinib
- 3rd generation TKIs: Ponatinib, Olverembatinib
- Specifically Targeting the ABL Myristoyl Pocket (STAMP) inhibitor: Asciminib
- Continue treatment indefinitely.
- Conception and pregnancy are contraindicated during TKI therapy.
- Imatinib mesylate
- Earlier known as STI-571
- Trial name: IRIS
- Dose: 400 mg orally OD. If there is no adequate response dose may be increased to 600-800mg/day.
- Take with meals with large glass of water
- Can cause Nausea, periorbital edema, rash, myalgia, headache, bone pain, fluid retention, hair repigmentation, muscle cramps, diarrhea
- Dose modifications:
- Hold if ANC <1,000/cmm or Platelet count <50,000/cmm and restart at dose of 400mg-OD once ANC >1500/cmm and platelet count >75,000/cmm.
- If same thing recurs, hold again till same time and restart at 300mg-OD. G-CSF may be used if required.
- Hold if Bilirubin>3xULN or AST/ALT >5xULN- Restart after normalization at reduced dose (300mg OD)
- Needs renal dose correction
- Hold if severe fluid retention and use diuretics, supportive care.
- Dasatinib
- Trial name: DASISION
- Dose: 50-100mg- OD Take medicine with meals.
- Optimal biologic dose (defined hypothetically as a dose that maintains the same efficacy but reduces toxicities) - 50mg OD
- Preferred in patients with history pancreatitis, elevated bilirubin or hyperglycemia.
- Avoid if patient has previous pleuro-pulmonary or pericardial diseases.
- Contraindicated mutations: T315I/A, F317L/V/I/C, or V299L
- If pulmonary arterial hypertension develops, discontinue permanently.
- Fluid retention/Pleural effusion/ Pericardial effusion to be treated with diuretics and supportive treatment +/- Prednisolone 40mg for 4 days, then 20mg for 4 days. When resolved reduce the dose (50mg/ 20mg-OD).
- Monitor BP, as hypertension is common.
- For rash use topical/ systemic steroids.
- Hematological toxicity: Hold if ANC <1000/cmm or PL count <50,000/cmm. Resume at same dose if recovery (ANC >1000/cmm and PL count >50,000) occurs within 1 week. If it takes longer time, restart at lower dose (80mgOD). If same episode recurs reduce the dose to 50mg-OD. Can use growth factors if needed.
- Causes QTc prolongation
- Can cause platelet dysfunction
- Switch to alternate TKI if: Pulmonary hypertension, Recurrent pleural or pericardial effusions despite dose reduction, Gastrointestinal bleeding, Immune-mediated adverse events (eg, colitis, pneumonitis, hepatitis, myocarditis, pericarditis, or nephritis)
- Nilotinib
- Trial name: ENEST-nd
- Dose: 150- 300mg- BD. Avoid food 2 hrs before and 1 hr after to avoid excess drug exposure.
- Dose de-escalation up to 200mg OD can be done if side effects occur.
- Useful in patients with lung disease, who have risk of developing pleural effusion.
- Contraindicated mutations: T315I, Y253H, E255K/V, or F359V/C/I
- Can cause hyperglycemia. Monitor sugars. Avoid in diabetic patients if possible.
- Can cause QTc prolongation, Hold if QTc>480msec.
- Can cause hypokalemia, hypomagnesaemia. So monitor regularly.
- Monitor BP, as hypertension is common.
- Hold if hepatic derangement and consider alternate therapies.
- Hold if ANC <1000 or Platelet- <50,000/cmm, resume at same dose once counts recover (ANC>1000 and PL>50K) within 2 weeks. Restart at reduced dose (400mg-OD) if recovery takes >2 weeks.
- Can cause coronary artery disease and rarely peripheral arterial occlusive disease.
- Can cause pancreatitis.
- Switch to alternate TKI if: Arterial and vascular adverse events, Recurrent pancreatitis despite dose reduction, Hyperglycemia, Immune-mediated adverse events (eg, colitis, pneumonitis, hepatitis, myocarditis, pericarditis, or nephritis)
- Bosutinib
- Trial name: BFORE
- Dose: Follow dose escalation strategy: 100mg- OD for 2 weeks, then 200mg- OD for 2 weeks, then 300mg- OD for 1 month, then if no side effects increase to 400mg- OD. Take medication with meal and large glass of water.
- Avoid in patients with inflammatory bowel disease or renal dysfunction
- Contraindicated mutations: T315I, V299L, G250E, or F317L
- Hold if ANC <1000/cmm or PL count <50,000/cmm. Resume at same dose if recovery (ANC >1000/cmm and PL count >50,000) occurs within 1 week. If it takes longer time, restart at lower dose (300mg-OD).
- Hepatic correction needed. If preexisting hepatic disease start at a dose of 200mg-OD.
- Hold if there is severe diarrhea and resume at 400mg-OD
- Fluid retention use diuretics and supportive care.
- Use topical or systemic steroids for rash.
- Hypertension is common.
- May cause pancreatitis
- Switch to alternate TKI if: Deranged LFT, Immune-mediated adverse events (eg, colitis, pneumonitis, hepatitis, myocarditis, pericarditis, or nephritis)
- Ponatinib
- Dose: Start with 45mg- OD, Decrease dose to 15mg-OD once IS is <1%. (For CMP AP/BC: Continue 45mg/day)
- Carries high risk of vascular events such as stroke and MI.
- Hematological toxicity: Hold if ANC <1,000/cmm or Platelet count <50,000/cmm and restart at dose of 300mg-OD once ANC >1500/cmm and platelet count >75,000/cmm.
- If same thing recurs, hold again till same time and restart at 15mg-OD.
- If same thing recurs, hold again till same time and restart at 10mg-OD.
- If same thing recurs, discontinue.
- G-CSF may be used if required.
- Switch to alternate TKI if: patient develops, arterial occlusive events/ heart failure, severe hypertension not responsive to antihypertensive medications, recurrent pancreatitis despite dose reduction
- Avoid with strong CYP3A4 inhibitors
- Asciminib
- Dose:
- CML-CP without TKI resistance mutation: 40mg- BD (In fasted state- Avoid food 2hrs before and 1 hr after)
- For patients with T315I mutation, the dose is 200mg-BD.
- Common side effects include fatigue, headache, nausea, arthralgia, diarrhea, thrombocytopenia, vomiting, rash, hypertension, pruritus and pain in extremities.
- In case of adverse effects decrease dose to 160mg- BD.
- Initially monitor once a week- CBC, SGPT, Bili, Creat, and ECG (For QTc).
- ANC- <1000/cmm or Platelet count <50,000/cmm- Hold till ANC >1500/cmm and platelet count >75,000/cmm. If recovery takes more than 14 days, restart at reduced dose. If recovery happens within 14 days, restart at same dose.
- If SGOT/SGPT >3xULN- Hold the dose until recovery and restart at reduced dose.
- If creatinine >1.5xULN- Hold the dose.
- For all other side effects as well, dose needs to interrupted and restarted at reduced dose once side effect subsides.
- Contraindicated mutations: A337T, P465S, M244V, or F359V/I/C
- Switch to alternate TKI if: Severe hypertension not responsive to antihypertensive medications, Recurrent pancreatitis despite dose reduction
- Dose:
- Olverembatinib:
- Useful in in patients with T315I mutation
- Dose: 40mg- OD- On alternate days
- Common side effects include: skin hyperpigmentation, hypertriglyceridemia, proteinuria, and severe thrombocytopenia
- Omacetaxine/ Homoharringtonine
- Cephalotaxus alkaloid
- Protein synthesis inhibitor
- Dose:
- Induction: 1.25 mg/m² SC BID for 14 consecutive days q28 days; repeat q28 days until hematologic response achieved
- Maintenance: 1.25 mg/m² SC BID for 7 consecutive days q28 days; continue as long as clinically beneficial
- Effective even against CML with T315I mutation
- Acceptable toxicity profile
- Allogeneic stem cell transplantation
- Indications:
- Blast crisis/ accelerated phase at diagnosis/ during follow up
- Resistance due to T315I mutation and non-availability/unaffordability of Ponatinib/ Asciminib
- Intolerance to all TKI
- Resistance to all TKI
- Before advent of Imatinib, BMT was the treatment of choice.
- Factors in influencing survival
- Patient’s age
- Disease phase at the time of SCT
- Disease duration
- Degree of histocompatibility between donor and recipient
- Gender of donor
- Overall 3 year survival rate- 15-30%
- Monitor patient by RT-PCR for BCR-ABL transcripts in blood / bone marrow
- Treatment options for relapse after HSCT
- TKI+ Lymphocyte transfusions from original donor obtained by leukapheresis
- Omacetaxine
- INF- Alfa
- Hydroxyurea
- Second transplant using same / different donor
- Autologous transplant is not useful in CML due to high degree of tumor cell contamination.
- PBSC harvest may be done from patients with complete molecular remission. These may be used in autoSCT, if patient shows progression of disease, as contaminating cells are still sensitive to TKI.
- Indications:
Other treatment options:
- Hydroxyurea
- Dose- 20-40mg/Kg/day
- Given to patients with significant leucocytosis (>1lakh/cmm), systemic symptoms or symptomatic splenomegaly, while awaiting BCR-ABL results
- Ribonucleotidereductase inhibitor
- Targets mature myeloid progenitors
- Treatment continued indefinitely
- Leukocyte count starts to fall within days and spleen reduces in size
- All features of CML are reduced by 4-8 weeks (usual maintenance dose is 1-1.5 g /day)
- Side effects (in high dose) – Nausea, Diarrhea, rarely, oral ulcers, skin rash
- Indication- Cytoreduction is newly diagnosed patients
- Interferon alfa
- Reverses all features of CML in 70-80%
- Given to patients who are diagnosed with CML during pregnancy
- 5-15% show restoration of Ph negative hematopoiesis
- Dose-3-9 mega units/m2/day – SC /IM- 5 times a week
- Pegylated INF- alpha 2a- 180mcg- SC- Once a week
- Response is seen within 1-3 months
- Side effects – Flu like symptoms (Fever, shivering, muscle ache) rarely – lethargy, malaise, anorexia, weight loss, depression, autoimmune diseases such as thyrotoxicosis
- Busulphan
- Alkylating agent
- Started 8 mg/day
- Dose reduced as leukocyte count began to fall
- Homoharringtonine
- A semi synthetic plant alkaloid
- Enhances apoptosis of CML cells
- Treatment of unaffording patients with CML in lymphoid blast crisis:
- Weekly Vincristine with Prednisolone for 4 weeks, and if patient returns to CML CP (1/3rd patients achieve this), start 2nd generation TKI.
Supportive Care:
- Allopurinol- Given during initial phase to prevent hyperuricemia
- Prevention of cardiovascular episode
- Potential risk factors (eg, diabetes, hypertension, hyperlipidemia, smoking, estrogen use) must be identified and corrected in every patient
- Assessment of cardiovascular risk must be done at diagnosis
- Those with 10 year risk of >10% should be given atorvastatin 20mg- daily.
- Patients with known coronary artery stenosis (>50%) must be given Aspirin.
Discontinuation of TKI (Treatment Free Remission)
- This should be avoided in resource limited settings, where survival is the goal of therapy. Monitoring BCR-ABL levels proves to be more expensive compared to treatment itself. In case, patients have to receive 2nd line TKI, it is not often possible for them. If there is progression to accelerated phase or blast crisis, allogeneic transplant is also often not possible.
- Criteria for discontinuation (All must be met)
- Age >18 years
- CML in chronic phase, with no history of AP/BC.
- Low- intermediate sokal score at diagnosis
- On approved TKI therapy for at least 3 years
- Stable molecular response (IS- <0.01%) for more than 2 years. Documented on at least 4 tests, performed at least 3 months apart.
- Access to reliable quantitative PCR test, with test results available within 2 weeks.
- Monthly molecular monitoring for 1 year, 2 monthly on 2nd year, and then 3 monthly thereafter, lifelong.
- TKI must be promptly restarted once patient loses molecular response.
- Approximate 3-year TFR rates are 40-50%. This percentage increases with higher duration of deep molecular response. Discontinuation after >5 years of DMR results in a 5-year TFR rate of >80%.
Special Situations:
- Parenting:
- Male patient: Risk of adverse event is same as that found in unaffected population.
- Female patient: Pregnancy while on any TKI must be strongly discouraged due to high risk of fetal abnormalities. Imatinib should be stopped immediately after confirmation of pregnancy and it can be re-introduced in 2nd trimester after explaining risk of hydrops fetalis. If patient is not willing to take any risk, stop TKI and monitor BCR-ABL every month. If IS >1% during follow up, options of management include interferon and TKI. LMWH or aspirin are indicated if there is thrombocytosis.
- TKIs are secreted in breast milk. Hence avoid breast feeding.
Related Disorders:
- Juvenile CML
- Present with prominent lymphadenopathy
- Hepatosplenomegaly is less
- Dermal infiltrates with eczema is often seen
- Facial rash is seen
- More hemorrhagic manifestations
- Poor prognosis
- Chloroma
- It is localized subperiosteal leukemic tumor mass
- Most commonly found in skull
- They are seen both in AML and CML
- Cells contain porphyrin / myeloperoxidase which gives the tumor green color upon fresh incision (This color fades on exposure to light)
- Treatment is similar to primary disease (CML/AML)
Figures:
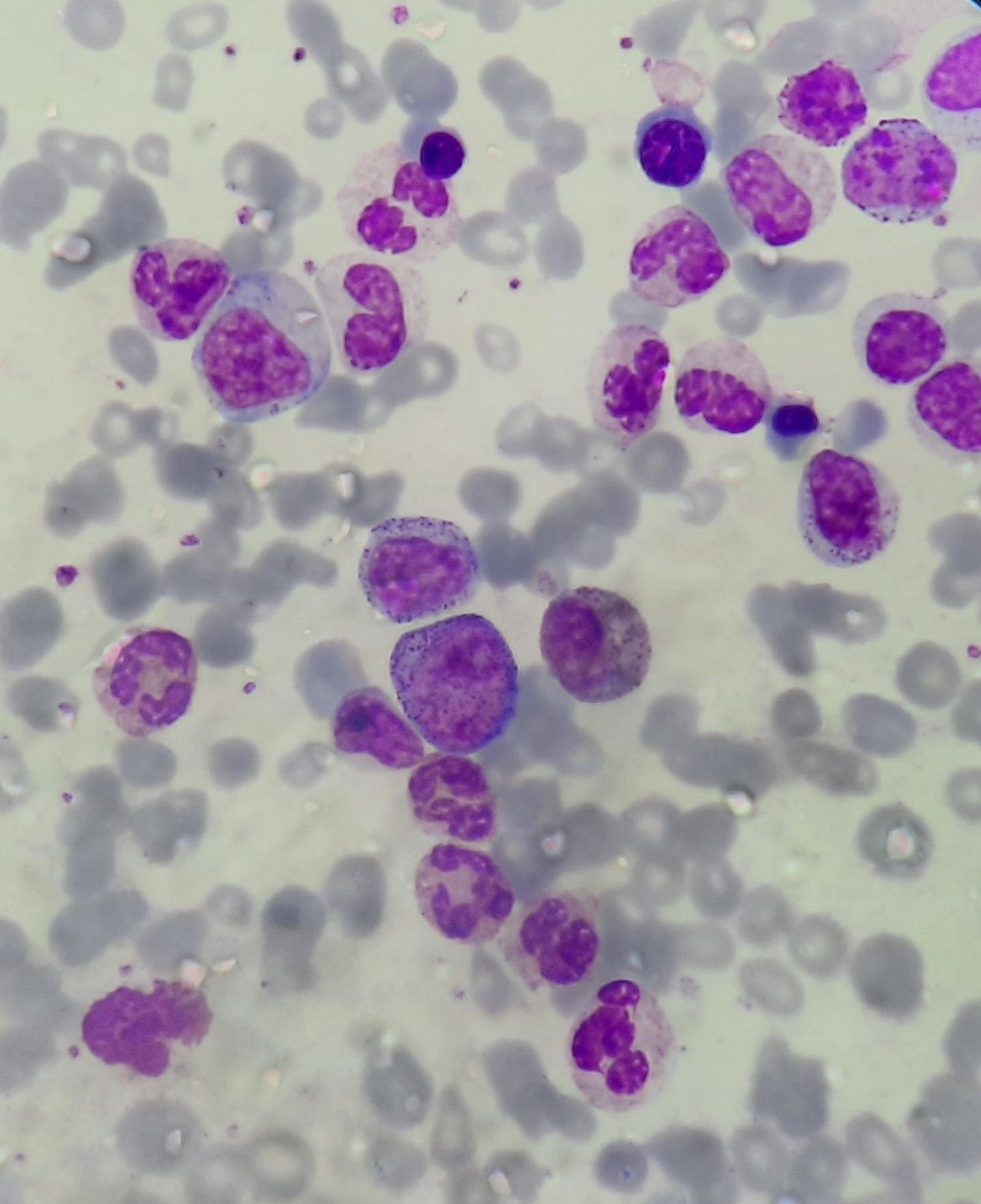
Chronic myeloid leukemia- chronic phase- Peripheral smear
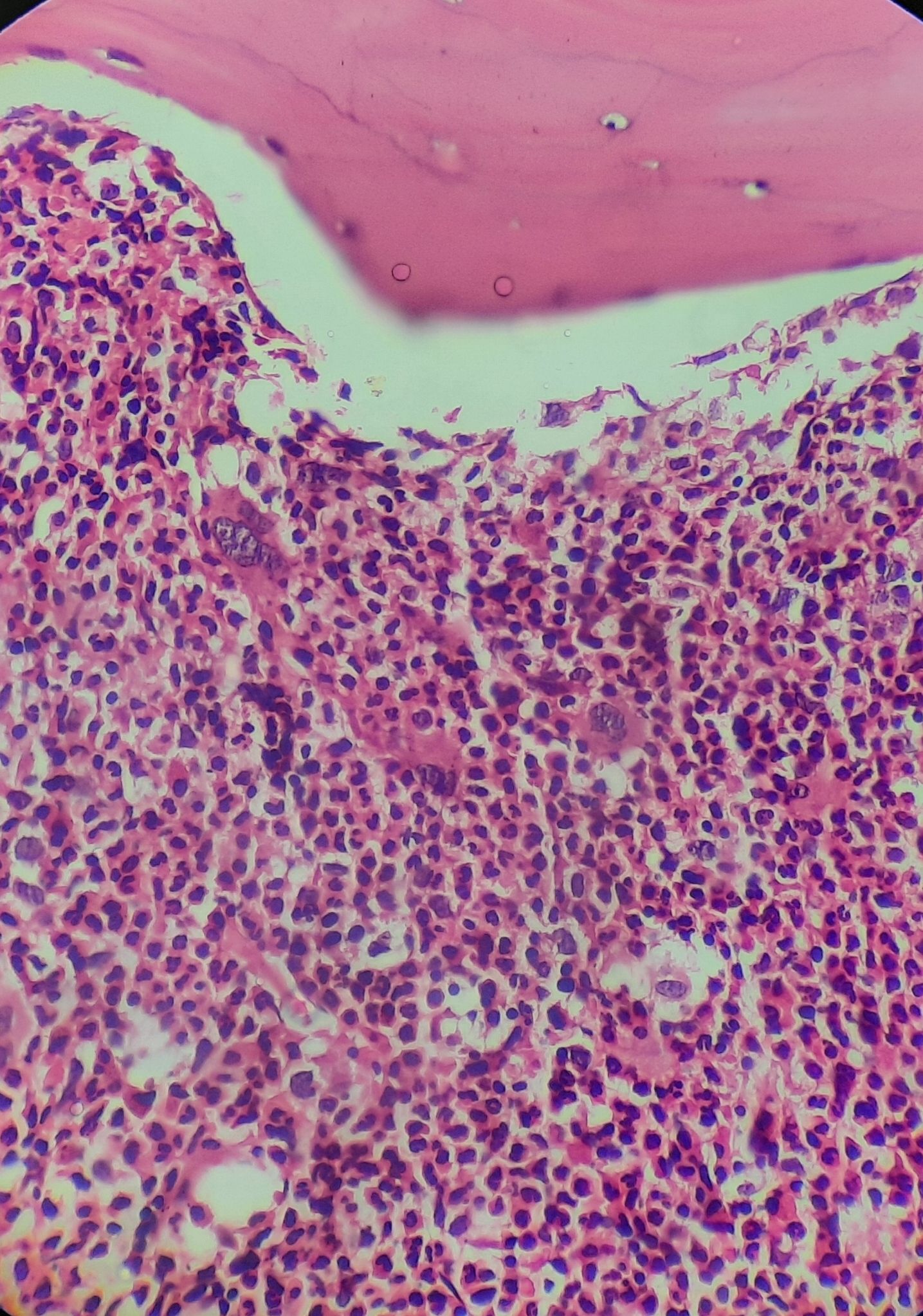
Chronic myeloid leukemia- chronic phase- Trephine biopsy
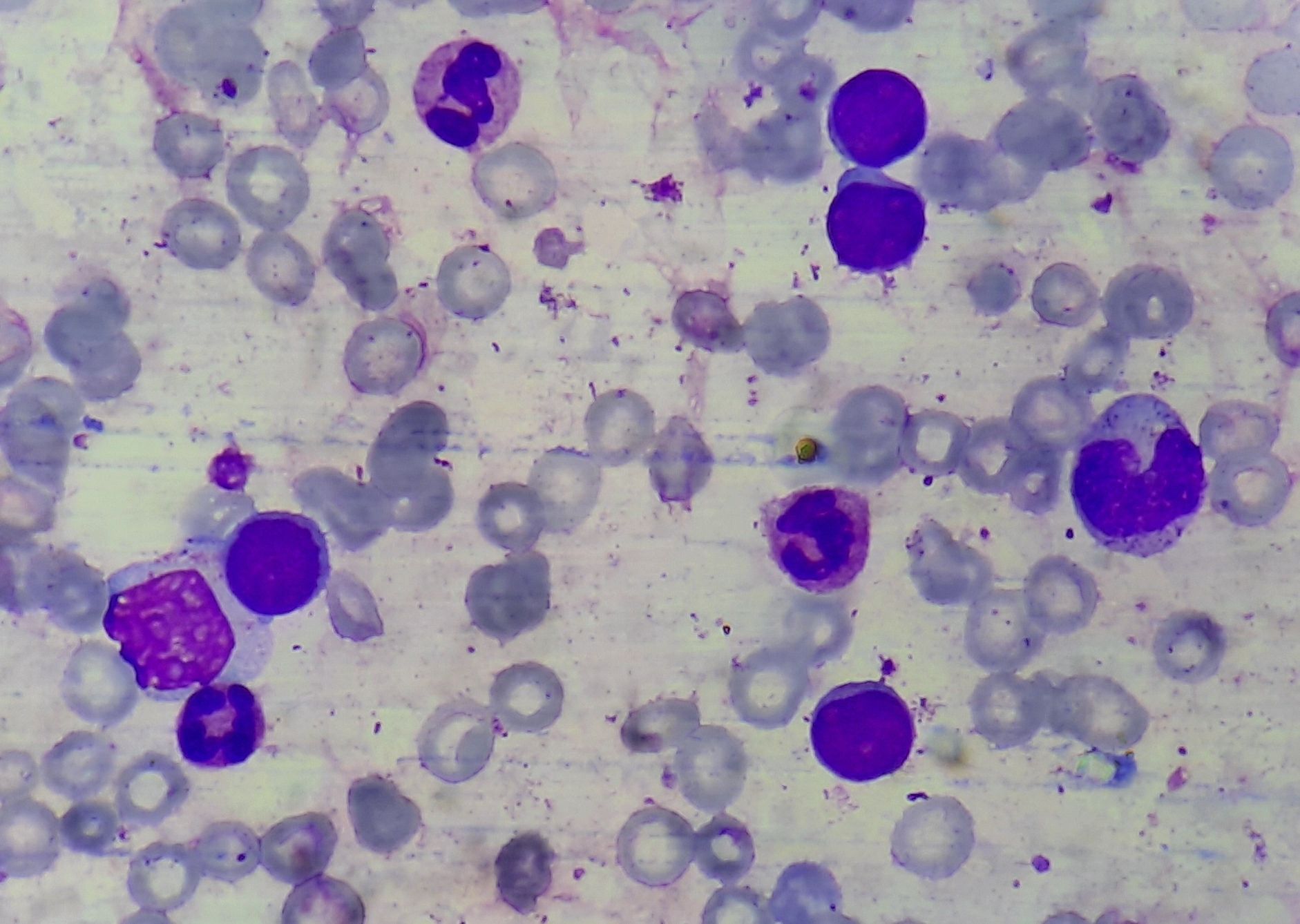
Chronic myeloid leukemia- blast crisis- Bone marrow aspirate
Recent advances:
Impact of additional genetic abnormalities at diagnosis of chronic myeloid leukemia
This study examined the impact of additional genetic abnormalities (AGA) at the diagnosis of chronic phase chronic myeloid leukemia (CP-CML) in a cohort of 210 patients treated with imatinib. AGA, including variants in cancer-related genes and novel rearrangements involving the Philadelphia chromosome, were identified in 31% of patients. Potentially pathogenic variants in cancer genes were found in 16% of patients, while structural rearrangements involving the Philadelphia chromosome were present in 18%. The analysis showed that patients with AGA had poorer molecular response rates and higher treatment failure rates despite proactive treatment intervention with imatinib.
https://doi.org/10.3324/haematol.2022.282184
Nilotinib and interferon-alpha in patients with chronic myeloid leukemia
The study investigates the impact of adding interferon-alpha to tyrosine kinase inhibitors in chronic-phase chronic myeloid leukemia patients, aiming to improve deep molecular response and treatment-free remission rates. The research, conducted within the ALLG CML11 trial, involved 12 patients receiving nilotinib and IFN-α and 17 patients receiving nilotinib alone. Key findings include a transient reduction in natural killer cell counts with nilotinib+IFN, but without affecting NK cell function. Additionally, IFN enhanced cytotoxic T-lymphocyte (CTL) responses to leukemia-associated antigens and influenced immune modulation, potentially impacting DMR and long-term outcomes. Further investigation into these immunological changes is warranted.
https://doi.org/10.1111/bjh.18984
Final 5-year analysis of DASFREE study
In the DASFREE study, patients with chronic myeloid leukemia in the chronic phase (CML-CP) who achieved a sustained deep molecular response (DMR) were eligible to discontinue dasatinib treatment and attempt treatment-free remission (TFR). After a 5-year follow-up of 84 patients who discontinued dasatinib, the 5-year TFR rate was 44%. No relapses occurred after month 39, and patients who did relapse and resumed dasatinib quickly regained a major molecular response in approximately 1.9 months. The most common off-treatment adverse event was arthralgia (joint pain), and nine patients experienced 15 withdrawal events. This study suggests that dasatinib discontinuation can be a viable and potentially long-term option for CML-CP patients with a sustained DMR, with a consistent safety profile.
https://doi.org/10.1111/bjh.18883
Treatment and follow-up of children with chronic myeloid leukaemia in chronic phase (CML-CP) in the tyrosine kinase inhibitor (TKI) era—Two decades of experience from the Tata Memorial Hospital paediatric CML (pCML) cohort
In a retrospective cum prospective study of pediatric chronic myeloid leukemia (pCML) in chronic phase, 173 children treated with imatinib were followed for long-term toxicities. Durable molecular response (DMR) was not attained in 34% of patients, with some requiring switch to second-generation TKIs due to poor response or mutations. Despite challenges, the 5-year event-free survival (EFS) and overall survival (OS) were high.
https://doi.org/10.1111/bjh.19251
European Stop Tyrosine Kinase Inhibitor Trial (EURO-SKI) in Chronic Myeloid Leukemia
The EURO-SKI study, the largest clinical trial on stopping tyrosine kinase inhibitors (TKIs) in chronic myeloid leukemia patients in stable deep molecular remission (DMR), found that 61% of patients maintained major molecular response (MMR) at 6 months, and 46% at 36 months. Key factors for predicting MMR loss at 6 months included TKI treatment duration and DMR before stopping TKIs. For late MMR losses, significant factors were TKI treatment duration, peripheral blood blast percentage, and platelet count at diagnosis. Overall, treatment duration, BCR::ABL1 transcript type, and peripheral blood blasts were identified as crucial for maintaining treatment-free remission.
https://doi.org/10.1200/JCO.23.016
Chronic myeloid leukemia diagnosed in pregnancy
Managing chronic myeloid leukemia (CML) during pregnancy is complex. An international registry supported by the European LeukemiaNet (ELN) reviewed 87 CML cases diagnosed in chronic phase from 2001–2022. Normal childbirth occurred in 76% of cases, with no increase in birth abnormalities or severe events. The low birth weight rate was 12%, similar to the general population. Elective and spontaneous abortions occurred in 21% and 3%, respectively. Imatinib achieved a 95% complete hematologic response rate before labor, compared to 47% with interferon-α. Interferon-α is viable in the 1st trimester, while imatinib and nilotinib are safe in the 2nd and 3rd trimesters.
https://doi.org/10.1038/s41375-024-02183-0
Long-term outcomes after upfront second-generation tyrosine kinase inhibitors for chronic myeloid leukemia
Second-generation tyrosine kinase inhibitors (2GTKI) are more effective than imatinib for first-line treatment in chronic myeloid leukemia in chronic phase (CML-CP), but failure still occurs. A retrospective analysis of 106 CML-CP patients treated with 2GTKI showed that 42.4% switched TKIs due to intolerance (26.4%) or resistance (16%). Most patients who continued 2GTKI achieved deep molecular responses (DMR), with 14.1% in treatment-free remission (TFR). Intolerant patients also achieved DMR with multiple TKI changes, while resistant patients had poorer outcomes, requiring advanced TKIs or alloSCT, and had significantly lower 7-year overall survival (66.1% vs. 100% and 97.9%; p=0.001).
https://doi.org/10.1038/s41375-024-02187-w
Asciminib in patients with CML with the T315I mutation: 2-year follow-up results
Asciminib, a novel tyrosine kinase inhibitor targeting the BCR::ABL1 myristoyl pocket, shows efficacy against BCR::ABL1T315I mutation, which is resistant to most approved therapies. In this phase I study of 48 heavily pretreated patients with T315I-mutated chronic-phase chronic myeloid leukemia (CML-CP), 62.2% achieved BCR::ABL1 ≤1% on the International Scale (IS). Major molecular response (MMR) was achieved by 48.9% of evaluable patients, including 34.6% of ponatinib-pretreated and 68.4% of ponatinib-naive patients. The most common grade ≥3 adverse events were elevated lipase (18.8%) and thrombocytopenia (14.6%), with 10.4% of patients discontinuing treatment due to adverse events.
https://doi.org/10.1038/s41375-024-02278-8
Second-generation tyrosine kinase inhibitors as first-line therapy in CML
This study explored changes in treatment and outcomes of chronic myeloid leukemia in the chronic phase (CP-CML) following the introduction of second-generation tyrosine kinase inhibitors (2G-TKIs) as first-line therapy. Patients treated before 2011 (imatinib era, n = 185) were compared with those treated after 2011 (2G-TKI era, n = 425), with dasatinib and nilotinib being the predominant therapies in the latter group. While progression-free survival, overall survival, and CML-related death (CRD) rates were similar between the two groups, the ELTS score more effectively predicted CRD in the 2G-TKI era, with high-risk patients experiencing better outcomes.
https://doi.org/10.1007/s12185-024-03758-4
Outcome of 3q26.2/MECOM rearrangements in chronic myeloid leukemia
This study aimed to evaluate outcomes in patients with 3q26.2/MECOM-rearranged chronic myeloid leukemia (CML). Among 55 patients, the median survival was 14 months, with a 5-year survival rate of 19%. Allogeneic stem cell transplantation showed improved survival (41% vs. 17% at 5 years). Multivariate analysis indicated that marrow blast percentage and achieving major cytogenetic response or deeper were predictors of survival.
https://doi.org/10.1007/s12185-024-03787-z
BCR::ABL1 kinase N-lobe mutants confer moderate to high degrees of resistance to asciminib
Secondary kinase domain mutations in BCR::ABL1, commonly causing resistance to ATP-competitive tyrosine kinase inhibitors (TKIs), are a significant challenge in treating chronic myeloid leukemia. Asciminib, a novel allosteric TKI, targets a regulatory pocket rather than the ATP-binding site, making it theoretically unaffected by ATP affinity changes. However, mutations like BCR::ABL1 M244V, located in the N-lobe, unexpectedly confer resistance to asciminib despite not affecting its binding. Molecular dynamic simulations suggest that M244V stabilizes an active kinase conformation, providing a new resistance mechanism that may impact asciminib-based combination therapies.
https://doi.org/10.1182/blood.2023022538
Asciminib in newly diagnosed chronic myeloid leukemia
In a phase 3 trial involving patients with newly diagnosed chronic myeloid leukemia (CML), 201 were assigned to receive asciminib (80 mg once daily) and 204 to investigator-selected TKIs. At week 48, major molecular response rates were significantly higher in the asciminib group (67.7%) compared to the TKI group (49.0%). Asciminib also outperformed imatinib (69.3% vs. 40.2%), with 66.0% response in the second-generation TKI group. Adverse events of grade 3 or higher were less frequent with asciminib (38.0%) compared to imatinib (44.4%) and second-generation TKIs (54.9%).
https://doi.org/10.1056/NEJMoa2400858
Asciminib monotherapy as frontline treatment of chronic-phase chronic myeloid leukemia: results from the ASCEND study
The Asciminib Evaluation in Newly Diagnosed CML study demonstrated that asciminib, achieved high efficacy and tolerance in newly diagnosed chronic-phase chronic myeloid leukemia (CP-CML). With a median follow-up of 21 months, early molecular response (BCR::ABL1 ≤10% at 3 months) was achieved in 93%, and major molecular response by 12 months in 79%. Most patients remained on treatment, with low discontinuation rates for toxicity (6%). Molecular response 4.5 was seen in 53% by 24 months, and adverse events were minimal. These results support asciminib as an effective frontline option for CP-CML.
https://doi.org/10.1182/blood.2024024657
Safety and efficacy of flumatinib as later-line therapy in patients with chronic myeloid leukemia
Flumatinib is a second-generation tyrosine kinase inhibitor (TKI), which is currently approved for patients with CML in China. In present study, in a cohort of 336 patients, response rates were high, with 86.4% achieving complete hematologic response, 52.7% complete cytogenetic response, 49.6% major molecular response, and 23.5% deep molecular response. Outcomes were notably better in patients treated as second-line therapy without prior resistance to second-generation TKIs. Adverse events were tolerable and consistent with prior findings, highlighting flumatinib as a viable treatment option in this setting.
https://doi.org/10.1182/blood-2023-181456
Outcomes of chronic myeloid leukemia patients after therapeutic failure to conventional tyrosine kinase inhibitors and asciminib
This retrospective multicenter study assessed patients with CML who failed ≥2 conventional TKIs and asciminib. Among 19 patients, 8 discontinued asciminib due to intolerance and 11 due to resistance. Intolerant patients often resumed prior TKIs with adjusted dosing, achieving better survival rates (100%) compared to resistant patients (71%) and those in accelerated/blastic phase (25%). Stem cell transplantation offered the best outcomes for resistance cases, while ponatinib showed limited efficacy and significant adverse effects. Other agents like interferon or hydroxyurea showed poor responses, highlighting the need for effective therapies in this high-risk group.
https://doi.org/10.1007/s00277-024-05906-6
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.