howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Hodgkin Lymphoma
Classical Hodgkin’s Lymphoma
Introduction:
- It is a neoplasm derived from germinal centre B-cells composed of mononuclear Hodgkin cells and multinucleated Reed –Sternberg cells residing in an infiltrate containing a variable mixture of non-neoplastic small lymphocytes, eosinophils, neutrophils, histiocytes, plasma cells, fibroblasts, and collagen fibers.
- As RS cells are derived from B-lymphocytes, the term Hodgkin lymphoma is preferred over Hodgkin disease.
Hodgkin Lymphomas share following characteristics which differentiates them from NHL
- Presence of Reed Sternberg cells.
- Usually arise in supradiaphragmatic lymph nodes, preferably in cervical region.
- Usually, single group of lymph node is involved
- Orderly spread by contiguity
- Mesenteric nodes and Waldeyer’s ring are rarely involved
- Extra nodal involvement in uncommon
- Systemic manifestations like fever are common
- Majority of them manifest clinically in young adults.
Epidemiology:
- Incidence – 2-3/1 lac population / year
- Common in people with higher socioeconomic status
- Bimodal age curve with peaks at 15- 35 years and 50 – 60 years.
- Slightly more common in men
- Accounts for 10% of all lymphomas
Etiology:
- Viral infection
- EBV-especially causes mixed cellularity type
- HHV-6.
- Hereditary- 1% patients have family history of HL. Increased incidence is observed with HLA-A1.Cecreased incidence seen with HLA-A*02.
- Immunosuppressed states such as HIV and treatment with immunosuppressive medications.
- Genetic alterations of 9p24.1 encoding PD ligand 1 or 2 are present in 97% patients with classical HL.
Pathogenesis:
Destructive somatic IGgV mutations are seen in HRS cells. Hence they are thought to be germinal center B cells.
EBV infection
↓
Tumor cells express LMP1 (Latent Membrane Protein)
↓
LMP1 upregulates NF-KB and PD-L1
↓
Inhibition of FAS mediated apoptosis of tumor cells
↓
Acquisition of additional mutations such as:
- NF-kB pathway:
- TNFAIP3, NFKBIA, NFKBIA, REL
- Constitutively activated NF-kB pathway promotes proliferation and inhibits apoptosis of HRS cells.
- JAK/STAT pathway:
- SOCS1, PTPN1, STAT6, STAT3, CSF2RB
- Affects differentiation, proliferation, and survival of B lymphocytes
- Regulators of immune escape: MHC class 1 component B2M, and the MHC class 2 transactivator (C2TA).
- Other pathways: MAPK/ERK, AP1, PI3K/AKT, and NOTCH1.
Transcription factor oct 2 or its activator BOB1/OBF1 is absent in RS cell.
(Octamer motif is an important element in regulation of immunoglobulin promoter and enhancer function.)
↓
Lack of immunoglobulin production
Tyrosine kinase receptors that are aberrantly expressed include
- Platelet derived growth factor receptor alpha
- Constitutive activation of PI3K-AKT and extracellular signal regulated kinase pathway
- Activator protein 1 transcription factors
Role of cytokines and chemokines
- Presence of inflammatory cells is due to release of cytokines from RS cells.
- IL – 5 – Infiltration of eosinophils
- IL – 13, IL- 9, IL- 6, RANK-L – Autocrine effect on RS cell
- TGFβ – Fibrogenesis
- Growth factors (M-CSF, FGF-2)
- Chemokines (e.g., CCL17/TARC, CCL5): Activation of CD4+ regulatory and helper T cells and stromal cells (fibroblasts, mesenchymal stromal cells)
- Survival of RS cells depends on cytokines released from inflammatory cells in the background. Ex: CCL5, CCL17, CCL22 from Th cells
- Other cytokines released are IL2, 6, 7, 9, 10, GMCSF, lymphotaxin A, Eotaxin and C-C chemokine.
CD4+ T cell surrounding the HRS cells, anti-inflammatory M2 macrophages and secretion of immunosuppressive cytokines (IL10, TGFβ) help tumor cells in evading host immunity.
RS cells utilize immunosuppressive mechanisms to promote survival through programmed cell death 1 (PD-1) signaling pathway. PD-1 which is excessively expressed on the surface of RS cells, down regulates the activity of surrounding T cells.
Classification: Depending on morphology
- Nodular sclerosis HL
- Mixed cellularity HL
- Lymphocyte rich HL
- Lymphocyte depleted HL
Clinical Features:
- Lymphadenopathy
- Lymph nodes in neck, supraclavicular region and mediastinum are commonly involved
- Lymph nodes are painless, rubbery and freely mobile
- Painful upon ingestion of alcohol
- Mediastinal involvement leads to dry cough, shortness of breath, SVC syndrome and chest pain.
- Contiguous spread to adjacent lymph nodes is seen
- Hepatosplenomegaly
- B Symptoms: Fever (>38o C), drenching night sweats, unintentional weight loss of >10% of body weight in last 6 months and pruritus
- Fever: Two types
- Low grade
- High grade fluctuating (Pel Epstein fever): High grade fever for 1-2 weeks followed by an afebrile period of 1-2 weeks.It is often associated with drenching night sweats
- Weight loss – more than 10% over 6 months
- Pruritus and skin rash
- High grade fever, sweating, pruritus and weight loss are called as B symptoms. 40% of patients have B symptoms
- Fatigue, malaise
- Rarely these patients may have
- Paraneoplastic cerebellar degeneration- Peripheral neuropathy, Guillain Barre syndrome, multifocal leukoencephalopathy
- Skin lesions such as erythema nodosum, icthyosiform atrophy and cutaneous infiltration forming multiple discrete, non-tender nodules.
- Nephrotic syndrome.
- Immune hemolytic anemia and thrombocytopenia.
- Hypercalcemia
- Bone pain
- Spinal cord and nerve root compression.
- Vanishing bile duct syndrome and idiopathic cholengitis
Investigations:
- Lymph node biopsy
- Gross
- Enlarged, and encapsulated
- Earlier they are discrete and later on are matted together
- Lymphocyte predominant – Homogeneous, fish- flesh like
- Nodular sclerosis- Nodular due to scarring
- Mixed cellularity and lymphocyte depleted – Abundance of necrosis.
- Reed Sternberg cell
- Their presence is characteristic of Hodgkin Lymphoma
- They are derived from Germinal center B lymphocytes, but lack B cell receptors and B cell associated genes and proteins.
- They comprise of 0.1-10% of total cells.
- They are large measuring 15-45 µm in diameter
- They are binucleated or have bilobed nuclei with two halves appearing as mirror image of each other. It has large, prominent, eosinophilic, inclusion like nucleolus which is surrounded by clear halo (“Owl eye” appearance).
- Cytoplasm is abundant and amphophilic
- Minimum requirement for diagnosis of RS cell is a bilobed nucleus in which at least one of the lobes has a prominent acidophilic nucleolus
- RS cell may be found in other conditions such as infectious mononucleosis, NHL etc.,
- Variants
- Popcorn cell – seen in NLPHL
- Hodgkin cell – Mononuclear variant of RS cell
- RS cell with pleomorphic hyperchromatic nuclei
- Mummified / necrobiotic variant – Darkly stained and retracted nuclei (seen because of apoptosis)
- Egg basket type RS cell – Has multilobed nucleus
- Lacunar cell – seen in nodular sclerosis HL
- Nodular sclerosis type HL
- It is most common form of HL worldwide, comprising of nearly 70% of all HL
- Microscopy shows lacunar cells. They are large cells having single hyperlobated nucleus, with multiple small nucleoli and abundant pale staining cytoplasm. The “frail” cytoplasm of these cells is retracted close to the nuclear membrane so that cell appears to be floating in a “lacuna”. This occurs because cytoplasm shrinks in formalin fixed tissue forming lacuna. No shrinkage is observed if tissue is fixed in Zenker’s fluid / B-15 fixative
- Extensive collagen bands are seen which divide lymph node into nodules.
- Diagnosis of nodular sclerosis is made even in absence of fibrosis, if typical lacunar cells are present – It is called cellular phase.
- Mixed Cellularity HL
- Most common form of HL in India
- Typical RS cells are seen in plentiful
- Fewer lymphocytes are seen, but background shows heterogeneous mixture of histiocytes, eosinophils, neutrophils and plasma cells
- Small areas of necrosis and fibrosis may be seen.
- Lymphocyte rich HL
- Comprises of 6% of all HL
- Shows diffuse infiltrate of mature lymphocytes with variable number of benign histiocytes
- Plenty of typical RS cells are present (This differentiates it from NLPHL)
- No necrosis or fibrosis seen
- Lymphocyte depletion HL
- It is rarest form of HL
- Microscopy shows very few lymphocytes but plenty of RS cells. There are many anaplastic, large pleomorphic cells
- 2 variants.
- Diffuse fibrotic variant – Hypocellular lymph node which is replaced largely by proteinaceous fibrillar material
- Reticular variant – It is much more cellular
- Gross
- Immunohistochemistry:
- RS Cells are usually positive for: CD-30 (almost all cases), CD – 15 in majority of cases (Paranuclear / diffuse cytoplasmic / cell membrane), Restin, fascin, PDL1 (40% cases), MUM1, PAX5 (95% cases, demonstrates B cells nature of HRS cells) and GATA3 (Nuclear expression)
- RS cells are usually negative for: CD-45, CD-20, CD19, T cell markers, Immunoglobulin, light chains, CD68R, CD138, OCT-2, BOB-1 and PU1.
- EBV (LMP-1) antigen/ EBER small nuclear transcripts- Positive in most of mixed cellularity type and lymphocyte depleted type of HL.
- Molecular studies
- Monoclonal immunoglobulin gene rearrangements
- Somatic mutations of immunoglobulin gene: Especially in variable region of Ig heavy chain genes. Indicates derivation of RS cells from germinal center B-cells.
- Blockage of apoptosis due to mutation of nuclear transcription factor NFKB
- EBV – mixed cellularity – 75% cases and nodular sclerosis – 10-40% cases.
- Cytogenetics
- Recurrent gains of the chromosomal sub-regions on chromosomal arms 2p, 9p & 12q
- Hemogram:
- Normocytic normochromic anemia
- Eosinophilia
- Lymphocytopenia
- Monocytosis
- Thrombocytosis
- ESR – Elevated
- Serum LDH levels –Elevated
- Chest-X-ray:
- Mediastinal mass ratio: Ratio of maximum width of mass and maximum intrathoracic diameter
- Ratio of >0.33 is defined as bulky disease
- Any mass measuring more than 10cm is also called bulky disease
- CT, MRI, gallium scan or Fluorodeoxy glucose PET scan
- 97-100% of HL are FDG avid
- Staging with PET CT is more accurate than CT alone
- Baseline scan is needed to assess response to therapy
- Bone marrow aspiration and biopsy –
- To note tumor cell infiltration
- Involvement indicates advanced stage of disease
- BM is involved in 5% of cases
- Staging laparotomy – Not done now. Following biopsies were taken
- Spleen
- Wedge of Liver
- Lymph nodes – Retroperitoneal, aortic, mesenteric
- Chip biopsy from iliac bone.
- After taking out all these biopsies, ovaries were placed behind the uterus so that they are not damaged during radiotherapy
- CD4-CD8 ratio – Reversed.
- HIV testing- Important, as antiviral therapies can improve disease outcome in HIV positive patients
- S. Calcium- Increased due to synthesis of 1, 25 dihydroxy vitamin D by RS cells
- S. Sodium levels- May be decreased due to SIADH
- S. Glucose- May be decreased due to auto-antibodies to insulin receptors
- Beta 2 microglobulin- Indicates tumor burden
Criteria for diagnosis:
- Essential:
- Primary nodal or mediastinal presentation.
- HRS cells and variants in a reactive microenvironment composed of varying proportions of small lymphocytes, eosinophils, histiocytes, plasma cells and neutrophils.
- Immunophenotype of HRS cells: CD30+, PAX5+ (weak to moderate), CD20-/ weak/ heterogeneous
- Desirable:
- Immunophenotype of HRS cells: CD15+, CD45-, decreased expression of OCT2 and BOB1
- EBV positive (~40% of cases)
- Histological subtyping (if possible)
- Exclusion of mimics of CHL by appropriate work-up (Infectious mononucleosis, EBV+ large B-cell lymphoma, Mediastinal grey zone lymphoma, Immune deficiency/dysregulation-associated lymphoproliferative disorders, Nodal TFH cell lymphoma, Peripheral T cell lymphoma, NOS, and anaplastic large cell lymphoma)
Staging (Ann Arbor with Cotswold revision)
- Stage I- Single lymph node region / single extra lymphatic site
- Stage II- Single lymph node region with extra lymphatic site or 2 or more lymph node regions on same side of diaphragm
- Stage III- 2 or more lymph node regions on both sides of diaphragm
- Stage IV- Diffuse involvement involving bone and liver
- Suffix
- A- No systemic symptoms
- B- Associated with weight loss, Drenching sweats & fever
- E- used for extra nodal involvement
- S- used for splenic involvement (Splenic involvement is considered as nodal disease, not as systemic spread)
- X- Bulky disease- A single nodal mass measuring at least 10cm in greatest diameter or greater than a third of the transthoracic diameter at any level of thoracic vertebrae
Prognosis:
- Overall, 5-year survival rate is > 95%
- Overall cure rates- >85%
- Favorable risk- Patients must have all of the following features
- Clinical stage I and II
- Maximum of three nodal areas involved
- Age < 50 years
- ESR < 50 mm/h without B symptoms or ESR < 30 mm/h withB symptoms
- Mediastinal / thoracic ratio < 0.35
- Unfavorable risk- Patients have any one of the following features
- Clinical stage II with involvement of at least four nodal areas
- Stage III or IV
- ESR > 50 mm/h
- Presence of B symptoms
- Presence of extranodal disease
- Bulky disease
- International prognostic score (Hasenclever Index): Used for advanced disease
- Factors included during scoring are:
- Age > 45 years
- Male gender
- Serum albumin < 4g/dL
- Hemoglobin level < 10.5 g/dL
- Stage IV disease
- Leukocytosis (white cell count > 15,000/cmm)
- Lymphopenia (<600/cmm or <8% of the white cell count)
- Each factor reduces predicted 5-year freedom from progression rate by approximately 8%
- Factors included during scoring are:
Risk factors | Freedom from progression at 5 years (%) | Overall survival at 5 years (%) |
0 | 84 | 89 |
1 | 77 | 90 |
2 | 67 | 81 |
3 | 60 | 78 |
4 | 51 | 61 |
>4 | 42 | 56 |
- Other factors associated with poor prognosis
- Black race
- Multiple nodules in spleen
- Increased LDH
- Increased β2 microglobulin
- Increased serum CD30 and Soluble CD25
- Microscopic type- Lymphocyte depletion
- CD15 negativity – increased chance of relapses.
Pretreatment Work-up:
- History:
- B-Symptoms
- Alcohol intolerance
- Examination
- LN (# of sites):
- Spleen:
- Liver
- WHO P. S.
- BSA
- IHC
- Subtype
- BMA and Bx(if cytopenia and –ve PET)
- Hemoglobin
- TLC, DLC
- Platelet count
- ESR
- LFT: Bili- T/D SGPT: SGOT:Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K: Ca:Mg: PO4:
- Uric acid
- LDH
- HIV:
- HBsAg:
- HCV:
- UPT
- CECT (CAP)/ PET (skullbase to midthigh)
- Stage
- Bulky/ Non-Bulky
- Favorable/ Unfavorable
- Prognostic score
- ECHO: LVEF- %
- Stop smoking
- UPT
- Fertility preservation
- PFT incl DLCO (Avoid bleomycin if DCLO <50%)
- Pneumococcus, meningococcus, HIB vaccination if splenic RT is planned
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
Treatment Plan:
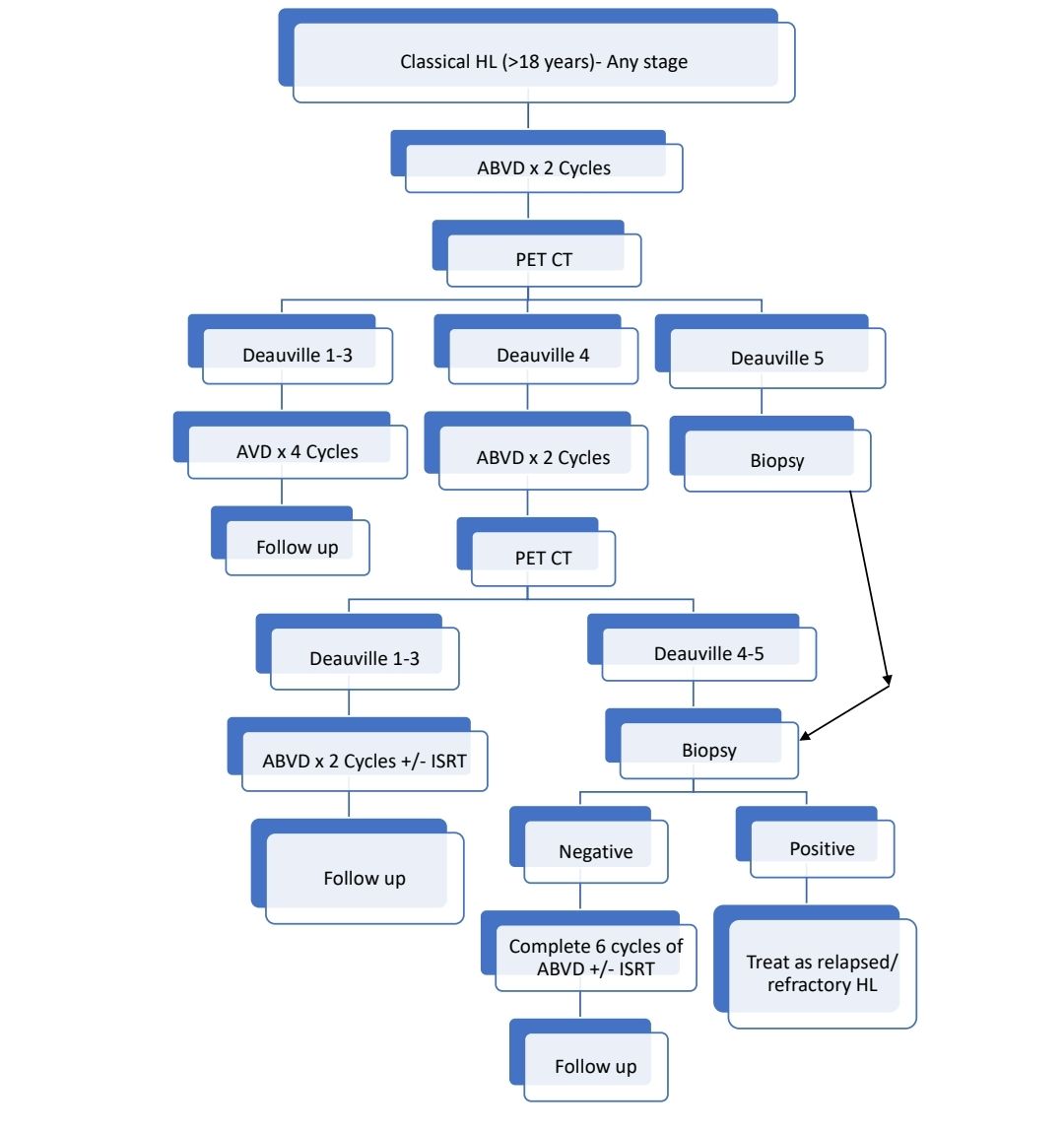
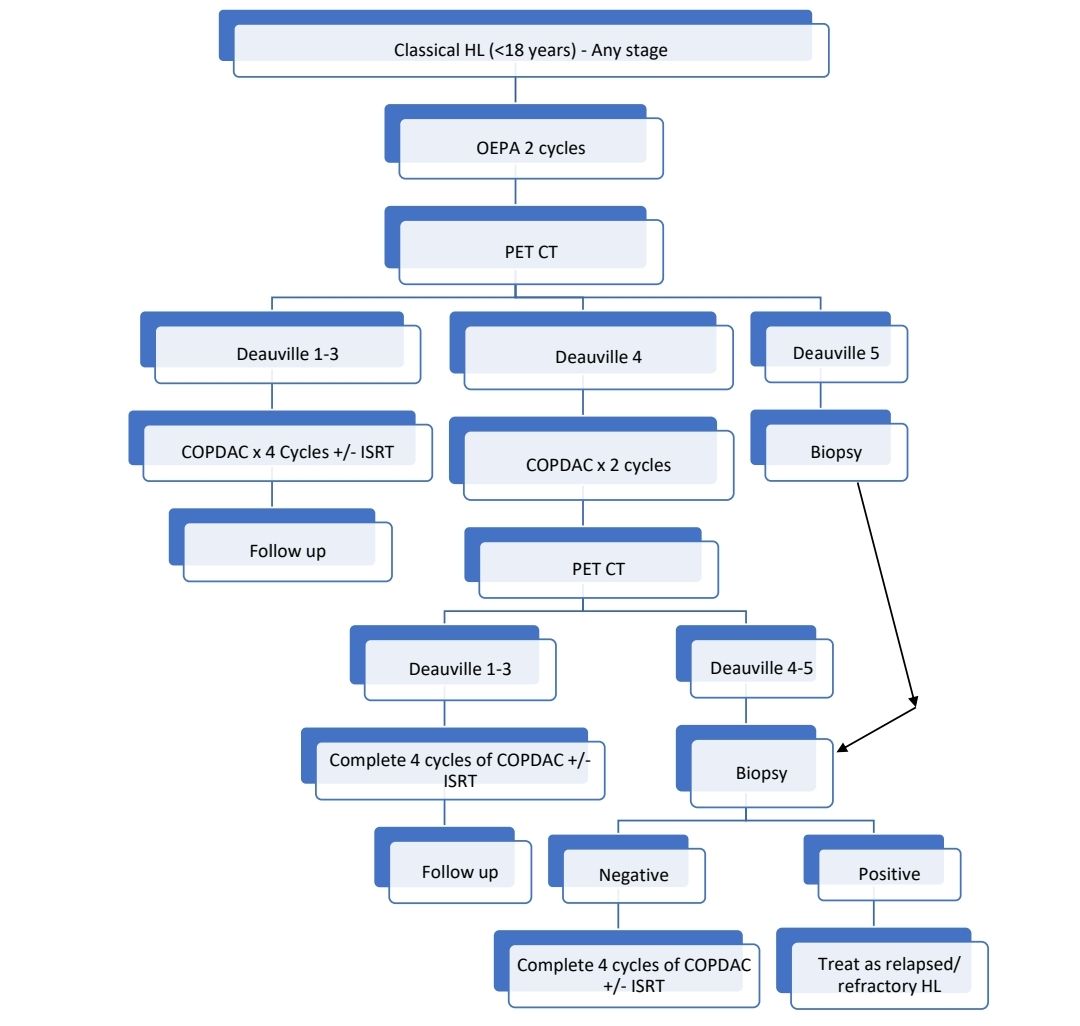
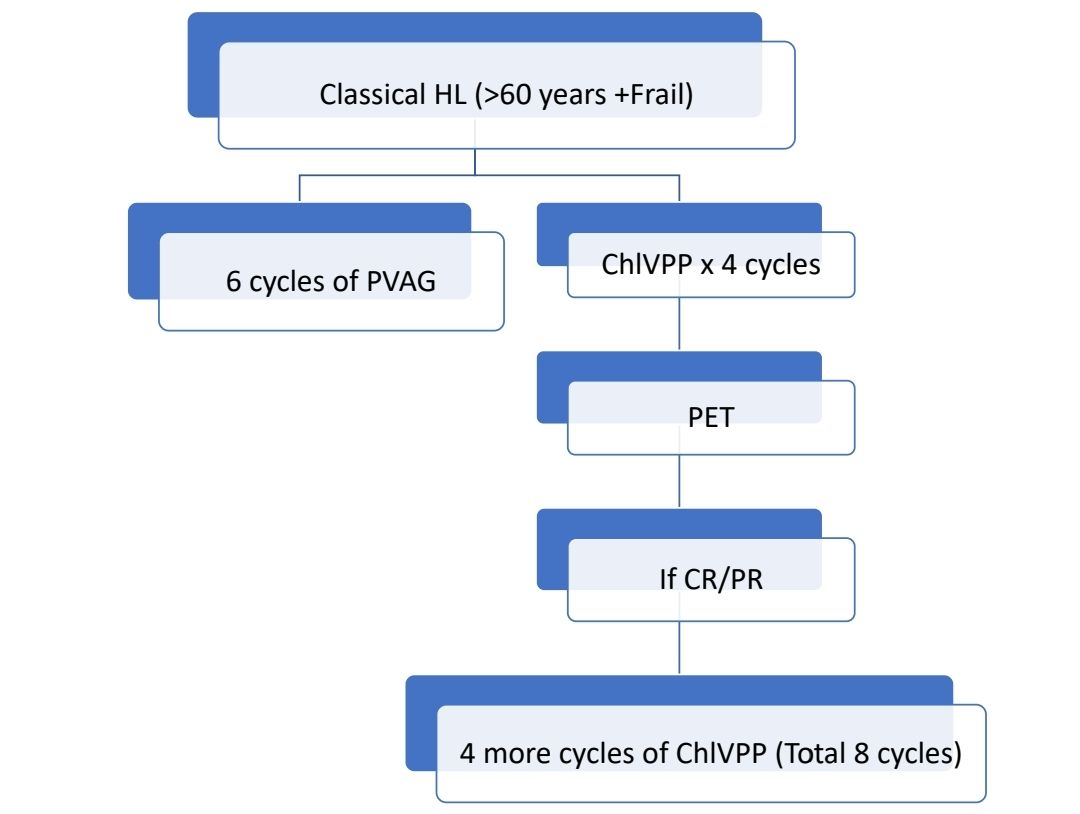
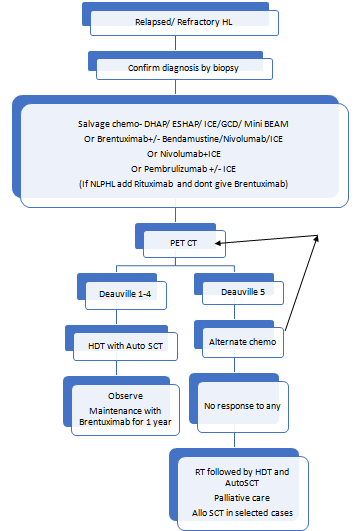
Other upfront treatment options include:
- Brentuximab+ AVD
- Escalated BEACOPP
- Nivolumab+AVD
- BrECADD (Brentuximab, etoposide, cyclophosphamide, doxorubicin, dacarbazine, dexamethasone)
For early stage favorable disease, 2 cycles of ABVD followed by 20Gy of ISRT can be done
If after 2 cycles of ABVD, PET CT is still positive, one can switch to BEACOPP
Response Criteria:
- Reporting of response (Deauville criteria)
- Score 1- No uptake
- Score 2- Uptake less or equal to mediastinum
- Score 3- Uptake greater than mediastinum but less than liver
- Score 4- Uptake moderately higher than liver
- Score 5- Uptake markedly higher than liver
- Score 1 and 2 are considered complete metabolic response (Negative scan)
- Score 4 and 5 are considered inadequate response to therapy (Positive scan)
- Score 3- It should be interpreted according to the clinical context
- After chemotherapy, stimulation of normal bone marrow may result in diffuse increased uptake which is higher than liver. Hence interim PET must be done as close to next cycle as possible.
- In case of positive scan, prior to starting 2nd line of therapy, biopsy must be done to exclude false positive uptake which can be sometimes because of treatment related inflammation.
- Timing of scan at the end of treatment
- Minimum 10 days after chemotherapy
- 2 weeks after G-CSF
- 3 months after radiotherapy
About Each Modality of Treatment
- ABVD
- Frequency: 14 days x2
- Inj. Dexamethasomne- 8mg- Stat
- Tab. Paracetamol- 500mg- Stat
- Inj. Doxorubicin 25mg/m2 (Max-50mg) in 100ml NS over 15 min
- Inj. Bleomycin 10 units/m2 in 100ml NS over 30 min
- Inj. Vinblastine 6mg/m2 (Max- 10mg) in 100ml NS over 15 min
- Inj. Dacarbazine- 375mg/m2 in 500ml NS over 3 hrs (Protect bag and tubing from light)
- Dose adjustments:
|
| Doxorubicin | Bleomycin | Vinblastine | Dacarbazine |
Platelet count (/cmm) | 75,000- 1lac | Give 50% of dose |
| Give 50% of dose |
|
| <75,000 | Delay chemo by 1 week | |||
ANC |
| At any ANC give same dose intensity. Do not give G-CSF | |||
Bilirubin (mg/dL) | 1.8-2.8 | Give 50% of dose |
| Give 50% of dose |
|
| 2.8-4.2 | Give 25% of dose |
| Give 25% of dose |
|
DLCO | <50% of predicted value |
| Discontinue |
|
|
Severe ileus | First episode |
|
| Delay until recovery, later give 75% of dose |
|
Ileus | Recurrent |
|
| Discontinue |
|
LVEF | <50% | Replace with Etopiside- 25mg/m2 on day 1, 50mg/m2 on day 2 and day 3 |
|
|
|
Peripheral neuropathy | Grade 2- Motor/ Grade 3 sensory |
|
| Give 50% of dose |
|
| Higher |
|
| Discontinue |
|
- ChlVPP
- Frequency: 28 days
- Cap. Chlorambucil- 6mg/m2- OD- From day 1 to day 14
- Tab. Procarbazine- 100mg/m2 (Max- 200mg)- OD- From day 1 to day 14.
- Tab. Prednisolone- 40mg/m2- OD – From day 1 to day 14.
- Inj. Vinblastine- 6mg/m2 (Max 10mg) in 100ml NS over 15min- On day 1 and day 8.
- Dose adjustments:
|
| Chlorambucil | Vinblastine | Procarbazine | Prednisolone |
TLC (/cmm)/ Platelet count (/cmm) | <3500 / <1lac
| Delay chemo by 1 week |
|
|
|
On Day 8 TLC (/cmm) and Platelet count (/cmm) | 81,000-99,000 and 3100-3490 | Give 67% of dose
| Give 50% of dose | Give 67% of dose
|
|
| 50,000-80,000 or 2,000-3,090 | Give 50% of dose | Give 50% of dose
| Stop |
|
| <50,000 or <2,000 | Omit | Omit | Omit |
|
Creatinine (mg/dL) | >2 |
|
| Omit |
|
Bilirubin (mg/dL) and SGPT/SGOT | 1.5-3 or 60-180 |
| Give 50% of dose |
|
|
| >3 and Normal | Omit | Give 50% of dose | Give 50% of dose |
|
| >3 and >180 | Omit | Omit | Omit |
|
Neurotoxocity | Grade 2 motor/Grade 3 sensory |
| Give 50% of dose |
|
|
| >2 Motor/ >3 Sensory |
| Omit |
|
|
- Brentuximab vedotin
- Anti CD30 antibody
- Commonly used as second or third line therapy
- Dose (in relapsed situation)- 1.8mg/kg- once in 21 days- for up to 16 cycles
- If given with AVD chemotherapy (ADCETRIS +AVD), 1.2mg/Kg-IV, on Days 1 and 15 with Cycle duration of 28 days, for total of 6 cycles (12 doses). Prophylactic G-CSF should be given to prevent febrile neutropenia.
- Side effects include- sensory neuropathy, neutropenia, and thrombocytopenia.
- OEPA
- Frequency: 28 days
- ½ DNS- 3,000ml/m2/day
- Tab. Prednisolone- 60mg/m2/Day in 3 divided doses- From day 1 to day 15
- Inj. Vincristine- 1.5mg/m2 (Max-2mg) in 100ml NS over 30min- on Day 1, Day 8 and Day 15 (3 days)
- Inj. Doxorubicin- 40mg/m2 in 100ml NS over 2 hrs- on Day 1 and Day 15 (2 days)
- Inj. Etoposide- 125mg/m2 in 250ml NS over 2hrs- From Day 1 to Day 5 (5 days)
- Dose adjustments:Start only if
- General condition is satisfactory
- WBC count >2000/cmm, ANC >500/cmm, Platelet count >80,000/cmm
- No contraindication to any of the prescribed drugs
- COPDAC
- Frequency: 28 days
- Tab. Prednisolone- 40mg/m2 in 3 divided doses- From day 1 to day 15
- Inj. Dacarbazine- 250mg/m2 in 100ml NS over 30min- From day 1 to day 3 (3 days)
- Inj. Vincristine- 1.5mg/m2 (Max-2mg) in 100ml NS over 30 min- On day 1 and day 8 (2 days)
- Inj. Cyclophosphamide- 500mg/m2 in 100ml NS over 1 hr- On day 1 and day 8 (2 days)
- ½ DNS- 3000ml/m2 for 24 hrs- On day 1 and day 8 (2 days)
- Inj. Mesna 200mg/m2- IV Bolus at 0, 4 and 8hrs- On day 1 and day 8 (2 days)
- Dose adjustments:Start only if
- General condition is satisfactory
- WBC count >2000/cmm, ANC >500/cmm, Platelet count >80,000/cmm
- No contraindication to any of the prescribed drugs
- Nivolumab
- Anti PD1 antibody
- Binding of PD1 (which is present of surface of cytotoxic T cells) to PD1 ligand, inhibits immune activation of cytotoxic T cells. Tumor cells circumvent T cell mediated cytotoxicity by expressing PD-L1.
- Dose: 240mg-IV in 250ml NS over 30min- every 2 weeks- Continue till disease progression or unacceptable toxicity
- No dose adjustments for renal/ hepatic impairment
- May cause pneumonitis/ colitis/ hepatitis/ nephritis. Use steroids. If pneumonitis/ colitis/ hepatitis/ nephritis is severe, permanently discontinue
- If Steven Johnson Syndrome/ Myocarditis/ Neurological toxicity/ Severe infusion reactions- Permanently discontinue
- Pembrolizumab
- PD1 inhibitor
- 200mg- IV- every 3 weeks- Continue till disease progression or unacceptable toxicity or 24 months without disease progression
- No dose adjustments for renal/ hepatic impairment
- Withhold further dose till recovery from hematological toxicity
- Permanently discontinue if there is severe pneumonitis/ severe hepatitis/ severe endocrinopathy (Ex: Hypophysitis/ hypo-hyper thyroidism)/ severe infusion reactions/ Steven Johnson Syndrome/ Severe nephritis/ severe colitis
- Radiotherapy
- 30-36Gy in 1.8 to 2 Gy fractions
- Generally given to patients who have bulky disease at diagnosis. Not necessary if end of treatment PET is negative. Must be given if there is residual PET-positive disease.
- Involved site radiation is preferred over involved field radiotherapy
- Avoided in recent times, due to concerns with respect to long term side effects (secondary malignancies, cardiac dysfunction).
- Autologous stem cell transplantation:
- Goal is to achieve long term disease control and a possible cure.
- Useful in patients with refractory or relapsed HL
- Must be done in patients with good performance score.
- BEAM is commonly used conditioning protocol.
- Other protocols used include:
- CBV (cyclophosphamide, BCNU, etoposide)
- Bu/Cy (busulfan, cyclophosphamide)
- Bu/Mel (busulfan, melphalan)
- IFRT may be done before or after ASCT, if is there is localized R/R disease. Care must be taken to exclude liver and lung.
- After ASCT consider Brentuximab vedotin/ PD-1 blockade maintenance
- Allogeneic stem cell transplantation:
- Indicated in medically eligible patients with R/R disease who were previously treated with brentuximab vedotin and autologous HCT.
- C-MOPP is generally used for adequate disease control prior to allo-SCT.
- RIC or Non- Myeloablative regimens are generally used for conditioning.
Supportive Care:
- Avoid use of G-CSF if bleomycin is being given, as it increases pulmonary toxicity.
- Bleomycin related pulmonary toxicity/ pneumonitis
- Seen in 1-3% of patients receiving bleomycin (30% in patients aged >60 years)
- Compromised renal function is major risk factor
- Presents with cough/ dyspnea with/ without fever)
- High index of suspicion is must
- Examination: Basal crackles
- CXR: Interstitial pattern of abnormality
- PFT: Decline in DLCO
- Treatment: Discontinue bleomycin and start high dose steroids.
- Give irradiated blood products during treatment and lifelong due to high risk of transfusion associated GVHD.
Late effects of HL and its Treatment
- Second malignancies- Most common is carcinoma of lung, Next is MDS/AML, Carcinoma of Breast.
- Cardiac disease- 3 times higher risk of MI
- Endocrine dysfunction- Hypothyroidism (in those who received neck RT), infertility
- Psychological trauma
- Hyposplenism
- Lung fibrosis- Due to use of bleomycin and radiation pneumonitis
Monitoring After Treatment/ Follow-up:
- Document complete remission by doing PET CT within 3 months of completion of treatment. (Do it between 2 and 3 months)
- Follow up once in 3-6 months with history, examination and labs (CBC, ESR, SGPT, Bili and Creat) for 3 years. Then once annually.
- TFT once a year if neck irradiation done.
- CT with contrast (C/A/P) only if clinically indicated. PET to be done only if CT is suggestive of relapsed disease as there is high chances of false positive results (Positive predictive value of PET/CT is only 28%).
- Cardiac stress test or echocardiogram once in 10 years.
- Surveillance for solid tumors (Ca skin, soft tissue, breast, lung) should begin 5-10 years following therapy and continued for life of patient.
- Annually give vaccination with H. Influenza, meningococcus, and influenza.
Special Situations:
- Special Care in Children
- Avoid laparotomy staging with removal of spleen to reduce the risk of fulminant sepsis.
- Risk adjusted therapy to avoid overtreatment of favorable risk patients.
- Avoid procarbazine in boys to protect future fertility
- Minimize radiation dose and field
- Minimize anthracycline dose.
- Hodgkin’s lymphoma in pregnancy:
- Priority must be given to health of mother
- Prognosis is same as other patients
- Must be co-managed by specialist obstetrician and fetal medicine unit
- Minimize fetal radiation during staging investigation and response evaluation. (Use MRI or USG)
- Delaying commencement of chemotherapy until post-delivery should be avoided as far as possible and should be done with extreme caution.
- Fetal risk is highest in the first trimester. Chemotherapy can be administered in the 2nd and 3rd trimester.
- ABVD is the regimen of choice. Dose is same as others.
- RT should be delayed post-delivery, wherever possible.
Nodular lymphocyte predominant HL (NLPHL)
Introduction:
- Germinal centre-derived B-cell neoplasm
- Tumor cells are surrounded by mantle zone B cells and follicular dendritic cells.
- Most commonly seen in early stage
- Infra-diaphragmatic nodes may be involved
- “Progressive transformation of germinal center” may co-exist or precede the development of NLPHL. It is associated with a chronic inflammatory or autoimmune disorder, which presents as unexplained, asymptomatic, persistent, or recurrent lymphadenopathy.
- T cell rich B cell lymphoma may coexist or develop in patients with NLPHL.
Epidemiology:
- Accounts for 5% of all HL cases
- 0.11 cases/1,00,000 people per year
- Male predominance (3:1)
- Median age: 30-40 years
Etiology:
- Mutations in PIM1, RhoH/TTF, PAX5, MYC, SOCS1, JUNB, DUSP2 and SGK1 genes
- BCL6 translocations are also seen
- Moraxella infection
Morphology:
- Nodal architecture is totally replaced by nodular/ diffuse infiltrate.
- Infiltrate consists of small lymphocytes, histiocytes, epithelioid histiocytes and intermingled LP (Lymphocyte Predominant) cells.
- Neoplastic LP cells are large
- Nucleus- Single, large, often folded so much that cell looks like “Popcorn” or “Elephant Feet”.
- Chromatin is mostly vesicular with thin nuclear membrane
- Nucleoli are multiple and basophilic.
- Cytoplasm- Scanty
- They are derived from antigen selected B cells
- Neutrophils and eosinophils are absent.
- Background consists of non-neoplastic B cells and T cells
- Background of large spherical meshwork of follicular dendritic cells. There are numerous small B cells and CD 57+ T cells.
Immunohistochemistry:
- LP cells are positive for
- Pan B markers: CD 20, CD79a, PAX-5, PU1, BOB1 and OCT-2.
- Germinal centre B-cell markers: BCL6, LMO2 and HGAL
- CD45, MEF2B
- They are negative for CD19, CD10, CD3, CD15, CD30, EBV (EBER), STAT6
- They are surrounded by resetting follicular helper T cells which are positive for CD3, CD4, CD57, PD1, CD57, BCL6, CXCL13, ICOS and PD-1.
Criteria for diagnosis:
- Essential:
- Nodular architecture, at least focally LP tumour cells
- Immunophenotype: Uniform positivity for several B-cell antigens (usually CD20-positive). Immune microenvironment: Many small lymphocytes, histiocytes and follicular dendritic cells; no eosinophils.
- Desirable:
- Characteristic immunophenotype of LP cells (CD20, OCT2, bcl6) and of background TFH cells (PD1- positive) rosetting around tumour cells
Prognosis:
- Clinical course is more indolent and prognosis is favorable.
- 10 year overall survival- >90% and progression free survival- >75%
- Late relapses are more frequent (7.4%) compared to classical HL (4.7%).
- Transformation to DLBCL is seen in 3-5% of patients.
- Adverse prognostic markers:
- Advanced stage of disease
- Age- >45 years
- Spleen/ liver/ bone involvement
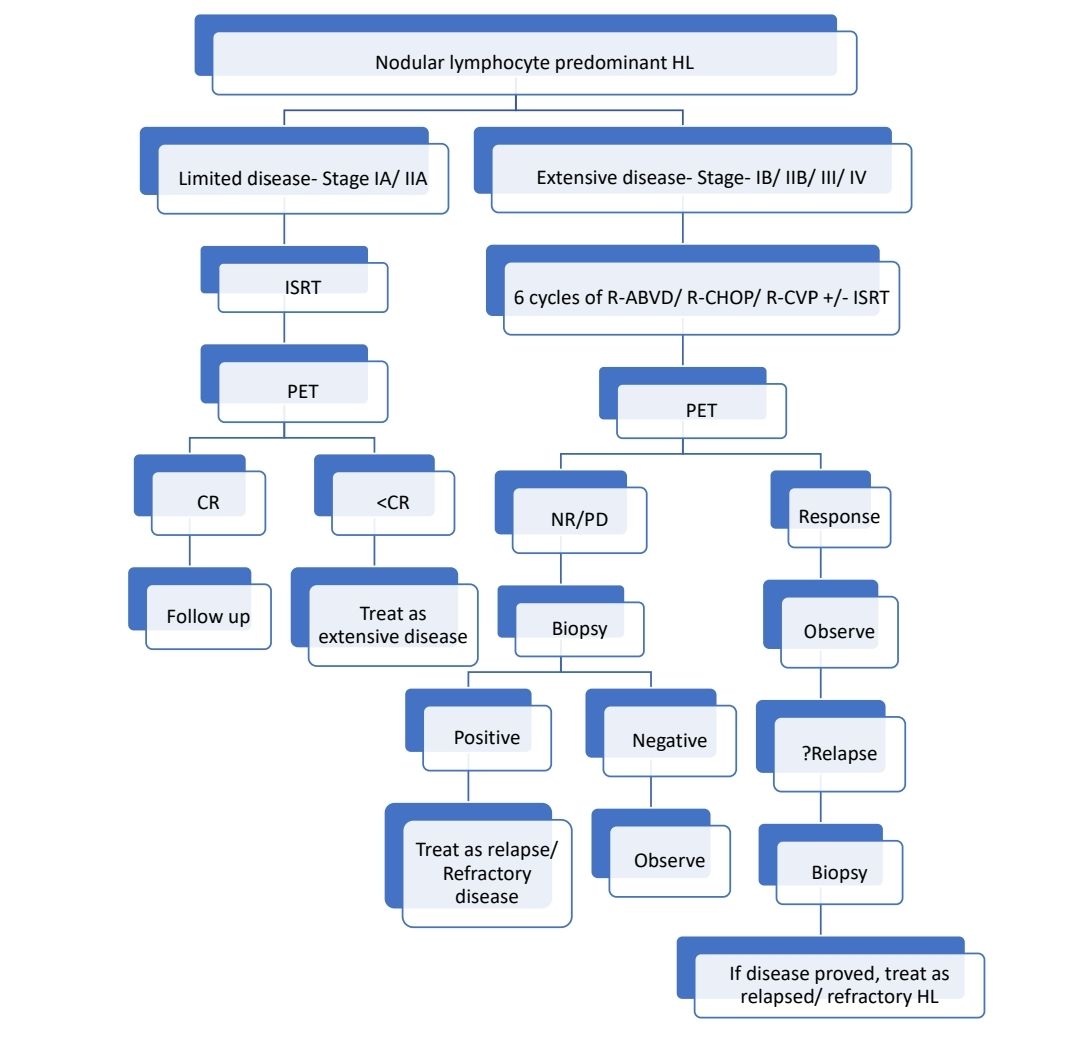
Treatment Plan:
Recent Advances:
Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma
In a retrospective study, Brentuximab vedotin, a CD30-directed antibody–drug conjugate, plus doxorubicin, vinblastine, and dacarbazine (A+AVD), was compared with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD). The 6-year overall survival estimates were 93.9% in the A+AVD group and 89.4% in the ABVD group. Fewer patients in the A+AVD group than in the ABVD group received subsequent therapy, including transplantation, and fewer second cancers were reported with A+AVD.
https://doi.org/10.1056/NEJMoa2206125
Long-term outcome of patients receiving haematopoietic allogeneic stem cell transplantation as first transplant for high-risk Hodgkin lymphoma
In this retrospective analysis from the Lymphoma Working Party-EBMT, it was observed that in patients with high-risk HL and candidates of allo-HSCT, a myeloablative conditioning strategy with T cell depleted graft might be the best option. 190 patients were included in this study, 63% of them had previously received brentuximab vedotin and/or checkpoint inhibitors.
https://doi.org/10.1111/bjh.17939
Brentuximab Vedotin Plus AVD for First-Line Treatment of Early-Stage Unfavorable Hodgkin Lymphoma: BREACH Trial
Present study assessed the efficacy and safety of BV-AVD in previously untreated, early-stage unfavorable Hodgkin lymphoma. After two cycles, the primary end point of the study was met: 82.3% patients in the BV-AVD arm were PET-negative compared with 75.4% in the ABVD arm. The 2-year progression-free survival (PFS) was 97.3% and 92.6% in the BV-AVD and ABVD arms, respectively.
https://doi.org/10.1200/JCO.21.01281
Risk Prediction Models for Coronary Heart Disease and Heart Failure After Treatment for Hodgkin Lymphoma
In the present study researchers used a multicenter cohort including 1,433 5-year HL survivors treated between 1965 and 2000 and age 18-50 years at HL diagnosis, with complete data on administered chemotherapy regimens, radiotherapy volumes and doses, and cardiovascular follow-up. Age at HL diagnosis, sex, smoking status, radiotherapy, and anthracycline treatment were included as predictors. This model can be used to identify HL survivors who might benefit from targeted screening for CVD.
https://doi.org/10.1200/JCO.21.02613
Positron Emission Tomography–Adapted Therapy in Bulky Stage I/II Classic Hodgkin Lymphoma
Patients with bulky stage I/II classic Hodgkin lymphoma (cHL) are typically treated with chemotherapy followed by radiation. Late effects associated with radiotherapy include increased risk of second cancer and cardiovascular disease. Present study tested a positron emission tomography (PET)–adapted approach in patients with bulky, early-stage cHL, omitting radiotherapy in patients with interim PET-negative (PET−) disease and intensifying treatment in patients with PET-positive (PET+) disease. The primary end point of 3-year progression-free survival (PFS) was 93.1% in PET2– and 89.7% in PET2+ patients. Three-year overall survival was 98.6% and 94.4%, respectively.
https://doi.org/10.1200/JCO.22.00947
Tislelizumab with gemcitabine and oxaliplatin in patients with R/R classic Hodgkin lymphoma
Tislelizumab (BGB-A317) is a humanized IgG4 anti–PD-1 monoclonal antibody . In this study, participants received induction therapy with gemcitabine, oxaliplatin, and tislelizumab followed by tislelizumab maintenance. The best overall response rate and complete remission rate were 100% and 96.7% respectively among 30 enrolled patients. The 12-month progression-free survival rate without transplant was 96%. Adverse events, mostly grade 1 or 2, included thrombocytopenia and anemia as common grade 3 or 4 events. Longer follow-up is needed to assess its curative potential.
https://doi.org/10.3324/haematol.2022.282266
Early-stage NLPHL- Utility of Interim PET
This study analyzed 100 patients with nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) in early stages treated in HD16 and HD17 trials. Treatment consisted of chemotherapy (HD16: 2× doxorubicin, bleomycin, vinblastine, and dacarbazine [ABVD]; HD17: 2× escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone [BEACOPP] plus 2× ABVD)and the omission of consolidation radiotherapy (RT) if interim positron emission tomography (iPET) was negative. Five-year progression-free survival (PFS) rates were 90.3% and 92.9%, similar to classical Hodgkin lymphoma patients within the same studies. In early-stage favorable NLPHL, those with negative iPET after chemotherapy tended to have worse 5-year PFS without consolidation RT compared to those with RT. While there were NLPHL recurrences, overall survival rate remained 100%.
https://doi.org/10.1182/blood.2023019939
Pembrolizumab monotherapy for relapsed/refractory classical Hodgkin lymphoma
The KEYNOTE-087 trial investigated pembrolizumabmonotherapy for relapsed or refractory classical Hodgkin lymphoma (cHL) patients. After over 5 years of follow-up, the study found that pembrolizumab demonstrated effective antitumor activity with acceptable safety. The overall response rate (ORR) was 71.4%, with 27.6% achieving complete response (CR) and 43.8% showing partial response. The median duration of response (DOR) was 16.6 months, and the median progression-free survival was 13.7 months. Notably, some patients maintained responses for over 4 years.
https://doi.org/10.1182/blood.2022019386
Long-Term Follow-Up of the Response-Adjusted Therapy for Advanced Hodgkin Lymphoma Trial
The long-term analysis of a response-adapted trial for adult patients with advanced-stage Hodgkin lymphoma aimed to confirm the noninferiority of treatment de-escalation by omitting bleomycin from doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) for interim fluorodeoxyglucose positron emission tomography (iPET)-negative patients. The study also assessed the efficacy and long-term safety for iPET-positive patients who underwent treatment intensification with escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (BEACOPP/BEACOPP14). With a median follow-up of 7.3 years, the 7-year progression-free survival (PFS) and overall survival (OS) for all patients were 78.2% and 91.6%, respectively. The results confirmed the noninferiority of treatment de-escalation after a negative iPET. For iPET-positive patients, treatment escalation with BEACOPP was effective and safe, with no increase in second malignancies.
https://doi.org/10.1200/JCO.23.011
Long-Term Follow-Up of the Response-Adapted Intergroup EORTC/LYSA/FIL H10 Trial for Localized Hodgkin Lymphoma
The final results of a preplanned analysis at a 10-year follow-up for the Early positron emission tomography (ePET) Response–Adapted Treatment in localized Hodgkin Lymphoma H10 Trial were reported. In ePET-negative patients, the 10-year progression-free survival (PFS) rates favored doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) with involved-node radiotherapy (INRT) compared to ABVD alone. In ePET-positive patients, the difference in PFS between standard ABVD and intensified bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPPesc) was not statistically significant. The analysis confirms that the omission of INRT in ePET-negative patients is associated with lower 10-year PFS, and BEACOPPesc intensification in ePET-positive patients was safe, with no increase in late adverse events.
https://doi.org/10.1200/JCO.23.0174
Brentuximab vedotin with dacarbazine or nivolumab as frontline cHL therapy for older patients ineligible for chemotherapy
In a phase 2 study, older patients (aged ≥60 years) with advanced-stage classical Hodgkin lymphoma (cHL) unfit for conventional chemotherapy received frontline treatment with brentuximabvedotin (BV) plus dacarbazine (DTIC) or nivolumab. For BV-DTIC, 95% achieved an objective response, with 64% achieving complete response (CR). Median duration of response (mDOR) was 46.0 months, median progression-free survival (mPFS) was 47.2 months, and median overall survival (mOS) was not reached at 63.6 months median follow-up. For BV-nivolumab, 86% achieved objective response, with 67% achieving CR. With 51.6 months median follow-up, mDOR, mPFS, and mOS were not reached. Treatment-emergent adverse events were manageable, with sensory peripheral neuropathy and neutropenia most common with BV-DTIC, and increased lipase, motor peripheral neuropathy, and sensory peripheral neuropathy most common with BV-nivolumab.
https://doi.org/10.1182/blood.2022019536
Prognostic Factors and Outcomes of Early-Stage Hodgkin’s Lymphoma
This study analyzed real-world data from five institutions in India on early-stage Hodgkin's lymphoma (ESHL) patients treated between 2000 and 2020. Among 258 evaluable patients, the median age was 37 years, with a majority being male (62%) and presenting with stage I disease (41%). The most common chemotherapies were ABVD (70%) and COPP-ABVD hybrid (21%), with a median of 4 cycles administered. After a median follow-up of 60 months, the 5-year event-free survival (EFS) was 87% and overall survival (OS) was 92%. Factors adversely affecting EFS included male gender and hemoglobin <10.5g/dL, while factors adversely affecting OS included hemoglobin <10.5g/dL, male gender, stage 2 disease, and ECOG PS (2–3). A 3-item prognostic score based on hemoglobin, stage, and gender could identify patients with very good or poor outcomes.
https://doi.org/10.1007/s12288-023-01692-9
Doxorubicin Exposure and Breast Cancer Risk in Survivors of Adolescent and Adult Hodgkin Lymphoma
This study of 1,964 female Hodgkin lymphoma (HL) survivors treated between ages 15-50 found a 1.5-fold increased breast cancer (BC) risk with cumulative doxorubicin doses >200 mg/m². After a median follow-up of 21.6 years, the 30-year cumulative BC incidence was 20.8%. The BC risk increase associated with doxorubicin was independent of age at first treatment or chest radiotherapy. These findings have implications for BC surveillance and treatment strategies in HL survivors.
https://doi.org/10.1200/JCO.23.013
Nivolumab+AVD in Advanced-Stage Classic Hodgkin’s Lymphoma
In a phase 3 trial, nivolumab with AVD (N+AVD) significantly improved progression-free survival (PFS) compared to brentuximab vedotin with AVD (BV+AVD) in patients aged 12+ with stage III or IV Hodgkin’s lymphoma. After 2.1 years of follow-up, the 2-year PFS was 92% for N+AVD versus 83% for BV+AVD (HR for disease progression or death, 0.45). N+AVD also had fewer adverse events, making it a promising option for treating advanced-stage Hodgkin’s lymphoma.
https://doi.org/10.1056/NEJMoa2405888
Low-dose nivolumab combinations in the management of relapsed/refractory Hodgkin lymphoma
A single-center study evaluated low-dose nivolumab (LD-Nivo) in 23 patients with relapsed/refractory classical Hodgkin lymphoma (r/r cHL), using monotherapy or combinations with brentuximab vedotin or chemotherapy. The study reported a 73% overall response rate, including 43% complete and 30% partial responses, with 1-year overall survival at 94.4% and progression-free survival at 89.4%. No significant differences in survival or efficacy were observed across LD-Nivo combinations or doses, with a median nivolumab dose of 0.78 mg/kg and a median of six cycles to achieve a response. Adverse events occurred in 61% of patients (none grade 3–4), suggesting LD-Nivo is a cost-effective and well-tolerated option for r/r cHL, warranting further research.
https://doi.org/10.1007/s00277-024-06098-9
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.