howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Immune Thrombocytopenia
Introduction:
- It is an acquired autoimmune disorder defined by low platelet count secondary to accelerated platelet destruction or impaired thrombopoiesis by antiplatelet antibodies.
Epidemiology
- 5.5/1lac population/ year
Etiology:
- Primary/ Idiopathic
- SLE, APLA
- Rheumatoid arthritis
- AIDS
- Immunoproliferative disorders like Hodgkin lymphoma, CLL, LGL leukemia etc.
- Following viral infections such as CMV, hepatitis, EBV, rubella, mumps, HCV etc.
- H. Pylori infection
- Auto immune thyroiditis, Autoimmune hepatitis
- Common variable immunodeficiency,Agammaglobulinemia, hypogammaglobulinemia, IgA deficiency
- Following bone marrow transplantation
- Post vaccination
- Drug related (Drugs act as haptens or sometimes they alter the antigen on platelet surface): Quinidine, quinine, gold salts, sulphonamides, heparin, penicillins, procainamides, alpha methyl dopa etc.
Pathogenesis:
1.
Auto antibodies (usually IgG type) against platelet membrane glycoprotein IIb – IIIa, Ib-IX or Ia-IIa
↓
Formation of antigen – antibody complex
↓
Acted upon by monocyte macrophage system especially in spleen via Fc receptors of macrophages
↓
Premature removal of platelets
2.
Autoantibodies bind to megakaryocytes
↓
Decreased platelet production
3.
Platelet antigens are presented with antigen presenting cells along with CD4 cells
↓
Stimulation of B cell clones to produce anti-platelet antibodies
4.
Epitope spreading- Peptides released from phagocytosed platelets are processed and presented to T cells
↓
T cells stimulate B cells to produce additional platelet auto-antibodies
Classification:
- Acute/Newly diagnosed- <3 months
- Persistent- 3-12 months
- Chronic- >12 months
Feature | Acute ITP | Chronic ITP |
Peak age incidence | Children, 2 to 6 years | Adult 20 to 40 years |
Platelet count | < 20 x 109 /L | 30-80 x 109/L |
Onset of bleeding | Abrupt | Insidious |
Antecedent infection (viral) | Commonly 1-3 weeks before onset | Unusual |
Sex predilection | None | Females 3:1 |
Eosinophilia and lymphocytosis | Common | More common |
Hemorrhagic bullae in mouth | Present in severe cases | Rare |
Spontaneous remissions | Occur in 80% of cases | Uncommon; course of disease fluctuates |
Clinical Features:
- Asymptomatic- Thrombocytopenia detected during routine evaluation
- Hemorrhagic manifestations such as
- Purpura/ Ecchymosis- Seen in areas exposed to pressure
- Hemorrhagic bullae in buccal mucosa (wet purpura)- Indicates severe thrombocytopenia and it is harbinger of life-threatening bleed
- Epistaxis
- Bleeding gums
- Malena
- Hematuria
- Menorrhagia
- Subarachnoid hemorrhage
- 70-80% of childhood ITP resolve spontaneously within weeks
Investigations:
- Hemogram-
- Isolated thrombocytopenia
- Other blood parameters are normal
- Sometimes
- Occasional atypical lymphocytes
- Eosinophilia
- Microcytic hypochromic anemia due to blood loss leading to iron deficiency
- Many megathrombocytes (platelets measuring more than 2-5 µm) are seen (It indicates accelerated thrombopoiesis). But these large platelets are not more than 30% of total platelet population. In case of hereditary macrothrombocytopenia, more than 60% of platelets are large and some are giant.
- Bone marrow examination
- See below for indications
- Megakaryocytes
- Number is increased up to 5 times. But decreased number does not rule out the diagnosis of ITP.
- Abundant cytoplasm with reduced cytoplasmic granularity and there is presence of vacuoles
- Single, large, nonlobated/ hypolobated nucleus
- Erythropoiesis and myelopoiesis are generally normal.
- PT and APTT-Normal
- Platelet count-Less than 1 lac/µL
- Anti-platelet IgG antibody detection on platelet surface- Not recommended as their levels are elevated even in other conditions causing thrombocytopenia
- Platelet survival study using 51Cr – Reduced to 2-3 days
- Hess tourniquet test – positive
- Sphygmomanometer cuff is inflated midway between systolic and diastolic BP.
- After 5 minutes, number of petechiae below cubital fossa are counted
- More than 20 petechiae indicates positive test.
- Blood TPO levels- Elevated
- DCT- Positive in Evan's syndrome
- Measurement of Immunoglobulins-
- To rule out CVID.If CVID immunosuppression must be used with caution.
- IVIg is contraindicated if there is IgA deficiency.
- Blood group- Rh typing is important if AntiD treatment is being considered
- HIV and HCV- As thrombocytopenia due to HIV and HCV are clinically indistinguishable from ITP
- H. Pylori testing- Urea breath test/ stool antigen test
Criteria for Diagnosis (International working group): Platelet count of <1,00,000/cmm, in absence of other causes or disorders that may be associated with thrombocytopenia. Presumptive diagnosis is made by exclusion i.e., after ruling out all other causes of thrombocytopenia.
- No positive findings in history- No Fever/ Bone pain/ Joint pain/ family history of low platelet/ risk factors for HIV infection/ childhood history of ecchymosis
- No positive findings in examination- No skeletal abnormalities/ lymphadenopathy/ splenomegaly
- Complete blood count- Normal except for thrombocytopenia
- Peripheral smear does not suggest other causes of thrombocytopenia, i.e. none of the following are seen
- Schistocytes
- Blasts
- Pseudothrombocytopenia- Platelet aggregation/ satellitism/ giant platelets
- Malaria or other parasites
- Response to IVIg almost confirms diagnosis of ITP, but it does not rule out secondary ITP.
- Bone marrow- If done no abnormality except for increased/ normal/ decreased megakaryocytes
Indications for bone marrow include
- Atypical presentation- fever etc
- Abnormality in physical examination- Lymphadenopathy/ splenomegaly
- Atypical features in peripheral smear
- Unresponsive to steroid/ IVIg therapy
- More than 60 years of age
- Those in whom splenectomy is considered
- If there is suspicion of underlying hematological disorder
Grading of disease severity:
- Mild- Few petechiae, Epistaxis which has stopped after local pressure
- Moderate- Numerous petechiae/ bruises. Epistaxis longer than 20min/ Other bleeding without hypertension/ fall in hemoglobin by >2gm/dl
- Severe- Epistaxis requiring packing, continuous bleeding leading to hypotension/ fall in hemoglobin to >2gm/dl
- Life threatening- Intracranial bleed or massive blood loss
Buchanan and Adix bleeding score
- Grade 0 (None)- None
- Grade 1 (Minor)- Few petechiae (<100) or Occasional bruises (<5)
- Grade 2 (Mild)- Many petechiae (>100) or >5 bruises
- Grade 3 (Moderate)- Overt mucosal bleed
- Grade 4 (Severe)- Internal hemorrhage (Brain, lung, muscle, joint etc)
- Grade 5 Life threatening/ fatal bleeding in any site
Prognosis:
- Risk of fatal bleeding is 0.0162-0.0389 cases per adult patient year.
- Risk of fatal bleeding increases exponentially after age of 40 years,
- Bleeding and treatment related immunosuppression, both equally contribute towards mortality.
Indications for Treatment:
- Adults: Platelet count <20,000/cmm with or without bleeding manifestations
- Children: Only if bleeding manifestations (mild skin petechiae should not be considered as bleeding manifestation), irrespective of platelet count i.e. even if platelet count is less than 20,000/cmm, if there is no bleeding manifestation, there is no need to treat. This rule applies only if, child can be closely monitored. If child cannot be closely monitored, it is better to treat if platelet count is <20,000/cmm.
- Those with thrombocytopenia, but no indications for treatment, must be
- Closely followed with serial monitoring of platelet count
- Advised to report if there is any bleeding manifestation
- Avoid contact sports and activities which can result in trauma
- Avoid NSAIDs
- Goal of therapy:
- To achieve platelet count that is associated with adequate hemostasis.
- Usual target is 30,000/cmm
Pretreatment Work-up:
- History
- Examination
- LN:
- Spleen:
- BMA and Bx (Indication: See above)
- Hemoglobin
- TLC, DLC
- Platelet count
- Peripheral smear
- PT
- APTT
- DCT
- Blood group
- LFT: Bili- T/D SGPT: SGOT:Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K:
- LDH
- HIV:
- HBsAg:
- HCV:
- H. Pylori testing
- Quantitative Ig: IgG: IgM: IgA:
- ANA by IF
- APLA workup
- UPT(If h/o amenorrhea)
- CMV PCR(Selected cases)
- Chest X Ray
- USG Abdomen
- NGS for Prinmary Immunodeficiency panel: Done in young patients with chronic ITP
Treatment Plan:
- Treatment must be tailored to individual patient taking into consideration following factors:
- Cost
- Co-morbidities
- Extent of bleeding
- Complications of therapy
- Activity and life-style
- Tolerance of side effects
- Accessibility to health care
- Patient expectations
In case of emergency (CNS/ GI/ Genito-urinary bleed or mucosal bleed with wet purpura)
- IVIg (If this is not possible- Emergency splenectomy)
- Initial treatment should include Inj. Methylprednisolone 1gm IV OD (for 2-3 days) supplemented with IVIg (1gm/Kg for 1-2 days)
- Platelet transfusions- 6hrly
- Vinca alkaloids
- Stop drugs causing platelet dysfunction
- PRBCs to maintain Hb>10gm%
- Tranexamic acid- If there is no hematuria
- Consider recombinant Factor VIIa
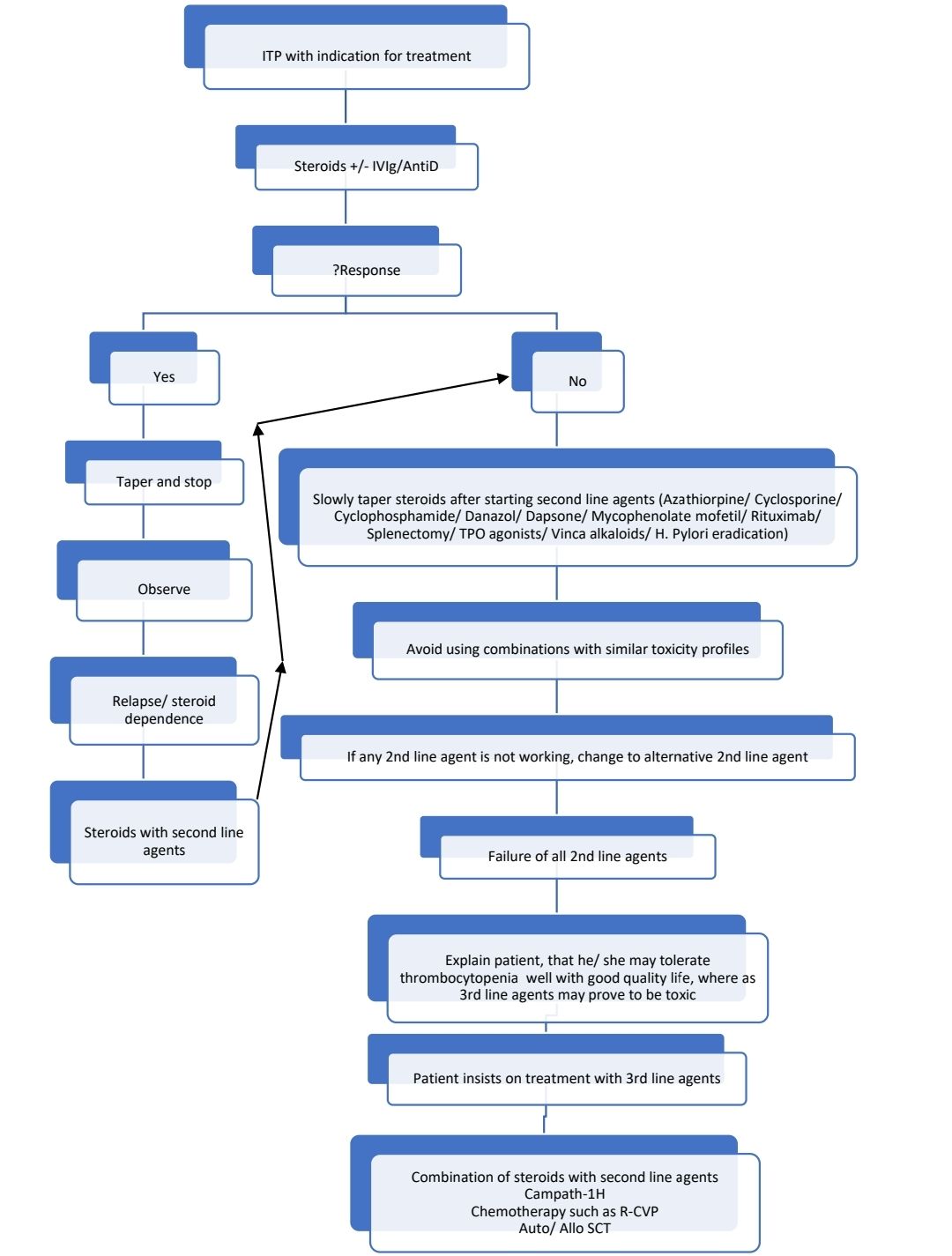
Response Criteria:
- Complete response: Platelet count >1lac/cmm on 2 occasions 7 days apart
- Response: Platelet count >30,000/cmm and greater than 2-fold increase in platelet count on 2 occasions 7 days apart
About Each Modality of Treatment:
- Steroids:
- Prednisolone- 1-2 mg/kg/d- till platelet count exceeds 50,000 / µL, then dose is gradually reduced over 4-6 weeks
- Alternatively, Inj. Methylprednisolone 30mg/kg/day can be given for initial 3 days, then switch over to Oral prednisolone.
- Shorter courses are preferred in children.
- Response rate- Adults: 70-80%, children- 80-90%
- 10-30% of adults have durable responses
- If there is no response after 3 weeks of steroids, then start 2nd line agent and start tapering steroids.
- Mechanism of action
- Inhibits interaction of immunoglobulin coated platelet with FC receptor of macrophages and inhibits phagocytosis.
- Decreases autoantibody production
- Improves bone marrow platelet production
- Produces stabilization of blood vessels, hence bleeding will stop even if platelet count is still low
- Steroid dependence: Need for ongoing or repeated administration of corticosteroids to maintain platelet counts in excess of 30,000/cmm and/or to avoid bleeding.
- Pulse Dexamethasone
- 40mg-OD for 4 days- such 4 day courses every 14 days for 4 cycles
- High risk of infection, hence should be avoided
- Response rate- 86% and sustained response in 50% patients
- Intravenous immunoglobulin (IVIg)
- Dose - 1 g/kg – IV Administered over 4-6 hours (single dose / two doses)
- Alternatively- 400mg/Kg- IV for 5 days.
- In both situations, further doses can be stopped if platelet count shows significant improvement.
- Given along with steroids for rapid rise in platelet count and reducing the risk of aseptic meningitis. But if steroids are contraindicated, IVIg can be used alone.
- Short time for response- Platelet count increases within 24 hrs. Hence useful in emergency setting, when patient has CNS bleed or GI hemorrhage.
- Improvement in platelet count is seen in 75% of patients and effect lasts for 3-4 weeks.
- Mechanisms of action:
- It blocks Fc receptor present on macrophages
- Anti-idiotype neutralization of anti-platelet autoantibodies
- Cytokine modulation
- Immunomodulation by suppressing T cells and decreasing auto-antibodies
- Complement neutralization
- Side effects – Headache, fever – chill reaction (sometimes anaphylaxis especially in patients with IgA deficiency), aseptic meningitis, renal failure, thrombosis, transmission of infections
- IV – Anti D
- Premedicate with paracetamol and steroids
- Given if patient is Rh positive who have not undergone splenectomy and who do not have history of AIHA.
- Dose 50- 75 µg/ Kg/ day for 4-5 days
- Response rate- Adults- 80%, Children- 50-80%
- Anti D coats Rh +ve cells which are removed by macrophages in spleen. In this process macrophages are saturated with RBCs, whose counts are high compared to number of platelets. Hence platelets are spared.
- Response is seen within 1 week of treatment
- Advantages
- Infused over shorter time
- Produced from small donor pool
- Potentially longer response
- Reduces the need for splenectomy
- Rituximab
- Has shown good response with less toxicity.
- Response rate- 60%
- Response may be seen after 1 month
- Durable and complete response in most of patients who respond
- Dose- 375mg/m2- weekly for 4 weeks.
- Alternatively 100mg-IV may be used
- Contraindicated in HBV positive patients, hence check HBsAg and Hepatitis B core antigen.
- Higher toxicity if given along with high dose dexamethasone
- Dapsone
- Dose- 100mg-OD
- Avoid in patients with G6PD deficiency
- Poor response in splenectomised patients
- Thrombopoietin receptor agonists:
- Useful because in chronic ITP despite severe thrombocytopenia TPO levels are not increased and reduced thrombopoiesis adds to low PL counts
- Response rate- 80%, but fall in platelet count is observed on stopping the treatment, hence taper and stop.
- May titrate the dose based on platelet counts
- Eltrombopag: 25-75mg-PO-OD- 2 hrs before or after meals (Children- Start with 1.25mg/Kg-OD and then increase the dose to target platelet count between 50,000 to 1,00,000/cmm)
- Romiplostim: 1-10microgm/Kg- SC- Once a week
- H. Pylori eradication
- This results in good improvement in platelet count
- Platelet improvement is seen even if this treatment fails to eradicate H. Pylori.
- Should be given to all patients who are found to have H. Pylori infection
- Includes 2 weeks course of
- Clarithromycin- 500mg- BD
- Amoxycillin- 1000mg- BD
- Pantoprazole- 20mg- BD
- Azathioprine:
- Dose- 150 mg/day- For about 18 months
- Should be continued for at least 4 weeks before its effect is evaluated.
- 45% response rate
- Vincristine –
- Dose- 0.03 mg/kg or 1 mg every week for 6 weeks
- Vinblastine-
- 10mg- every week for 3 weeks
- Danazol-
- Dose – 600 mg/day.
- Avoid until puberty as it has adverse effect on growth.
- Response rate- 60%
- Cyclosporine
- Dose- 2.5-3mg/kg/day in 2 divided doses
- Maintain trough levels- 100-200ng/ml
- Avoid in older patients and in those with renal insufficiency.
- 80% response rate
- Remissions are durable
- Cyclophosphamide
- Dose- 1-2mg/kg- OD for at least 16 weeks
- Response rate- 24-85%
- Mycophenolate mofetil
- Dose: Start with 250mg-OD, and then gradually increase to 1000mg/day
- Response rate- 78%
- Splenectomy
- Removes the site of destruction and source of antibody formation
- Deferred until at least 1 year from diagnosis and in children till child is > 5 years of age
- If platelet count is <30,000/cmm, prior steroids and IVIg must be given to raise the platelet count, so that there is minimal risk of surgical bleeding.
- Perioperative platelet transfusions may be needed if platelet counts are very low.
- Prophylactic vaccination and other precautions- Refer to splenectomy section
- Decision to do splenectomy depends on
- Severity of thrombocytopenia and bleeding
- Side effects of treatment
- Fitness for surgery
- Patient compliance with treatment
- Patient preference
- Nearly 80% of adults respond well to splenectomy initially. 2/3rd have sustained response.
- Stem cell transplantation
- High rates of complications including sepsis and cerebral bleeding, so not used
Management of secondary ITP:
- HCV associated ITP
- Start antivirals if there are no contraindications
- Antiviral therapy improves platelet count
- If interferon is given closely monitor platelet count, as interferon also can decrease platelet count.
- If ITP needs to be treated, IVIg is initial treatment of choice.
- Instead of steroids, better to give Eltrombopag as first line therapy (25-75mg-OD)
- Management should be done in consultation with hepatologist
- HIV associated ITP
- Start HAART as soon as possible
- Initial treatment should consist of IVIg with corticosteroids
- If refractory, splenectomy is preferred over other second line options.
- ITP secondary to primary immunodeficiency: Treatment as per the defect
- ALPS or PIK3delta activation syndrome- Rapamycin inhibitors
- CTLA-4- and LRBA deficient patients- CTLA-4 fusion protein therapy
- GOF variants in JAK1 or JAK2- JAK inhibitors
- NFATC1 variants- calcineurin inhibitors
Other Treatment Options:
- Newer agents:
- Oral Syk inhibitor- R788
- Syk is a downstream regulator of monocyte-macrophage phagocytosis
- Side effects- Diarrhea, nausea, emesis
- Anti-CD40L antibody (hu5c8 and IDEC-131)
- CD40 is critical for T cell dependent B cell expansion
- Response rate- 15%
- Oral Syk inhibitor- R788
Special Situations:
- Target platelet counts during surgery in adults
- Dental prophylaxis (descaling) and simple extraction- 30,000/cmm
- Complex extractions- 50,000/cmm
- Minor surgery- 50,000/cmm
- Major surgery- 80,000/cmm
- Major neurosurgery- 1,00,000/cmm
- Concomitant use of antifibrinolytics immediately prior to the procedure may be helpful.
- Pregnancy and ITP
- Go through "thrombocytopenia in pregnancy" in consultative hematology section. First rule out all other causes of thrombocytopenia.
- In contrast to gestational thrombocytopenia, ITP is seen usually in first trimester.
- Only about 30% of ITP during pregnancy need intervention
- Pregnancy is a prothrombotic state, hence there is less chances of bleeding
- Monitor platelet count regularly
- Treat only of platelet count falls to less than 30,000/cmm or bleeding manifestations.
- Maintain platelet count
- >30,000/cmm- Throughout pregnancy
- >50,000/cmm- For cesarian section or vaginal delivery (Mode of delivery should be based on obstetric indication)
- >80,000/cmm- For spinal/epidural anesthesia
- Treatment:
- Prednisolone- Start with 20mg OD- Adjust to a minimal dose that produces hemostatically effective platelet count.
- IVIg/ AntiD- When rapid increase in platelet count is required
- Second line-
- Azathioprine- Considered safe in pregnancy
- Consider laparoscopic splenectomy. It is best done in 2nd trimester.
- Avoid Rituximab as it inhibits fetal B cell development
- Platelet count in fetus may be reduced, hence
- Avoid fetal scalp electrodes/ fetal blood sampling
- Avoid ventous delivery and rotational forceps
- Avoid IM injections to newborn till platelet counts are known
- Do transcranial doppler if platelet count is <50,000/cmm
- Monitor platelet count daily, as nadir is reached usually between 2nd and 5th day of life.
- If platelet count is <20,000/cmm, give IVIg- 1gm/ Kg. One more may be repeated the following day if there is no improvement in platelet count.
- Transfuse platelets if there is life-threatening bleeding
- Fetal thrombocytopenia tends to worsen with subsequent pregnancies
Related Disorders:
- Evan’s syndrome
- Co-existence (either simultaneous or sequential) of ITP along with autoimmune hemolytic anemia
- Generally has chronic course
- Primary immunodeficiency is detected in 65% of patients, hence NGS for PID panel must be done in all cases
- Differential diagnosis that must be excluded prior to diagnosis of Evan's syndrome
- SLE
- IgA deficiency
- CVID
- AIDS
- ALPS
- Treatment
- First line: Steroids +/- IVIg
- Second line: Cyclosporibe, MMF, Vincristine, Danazol, Combination of these, Rituximab
- Third line: Splenectomy, Autologous stem cell transplantation
Figures:
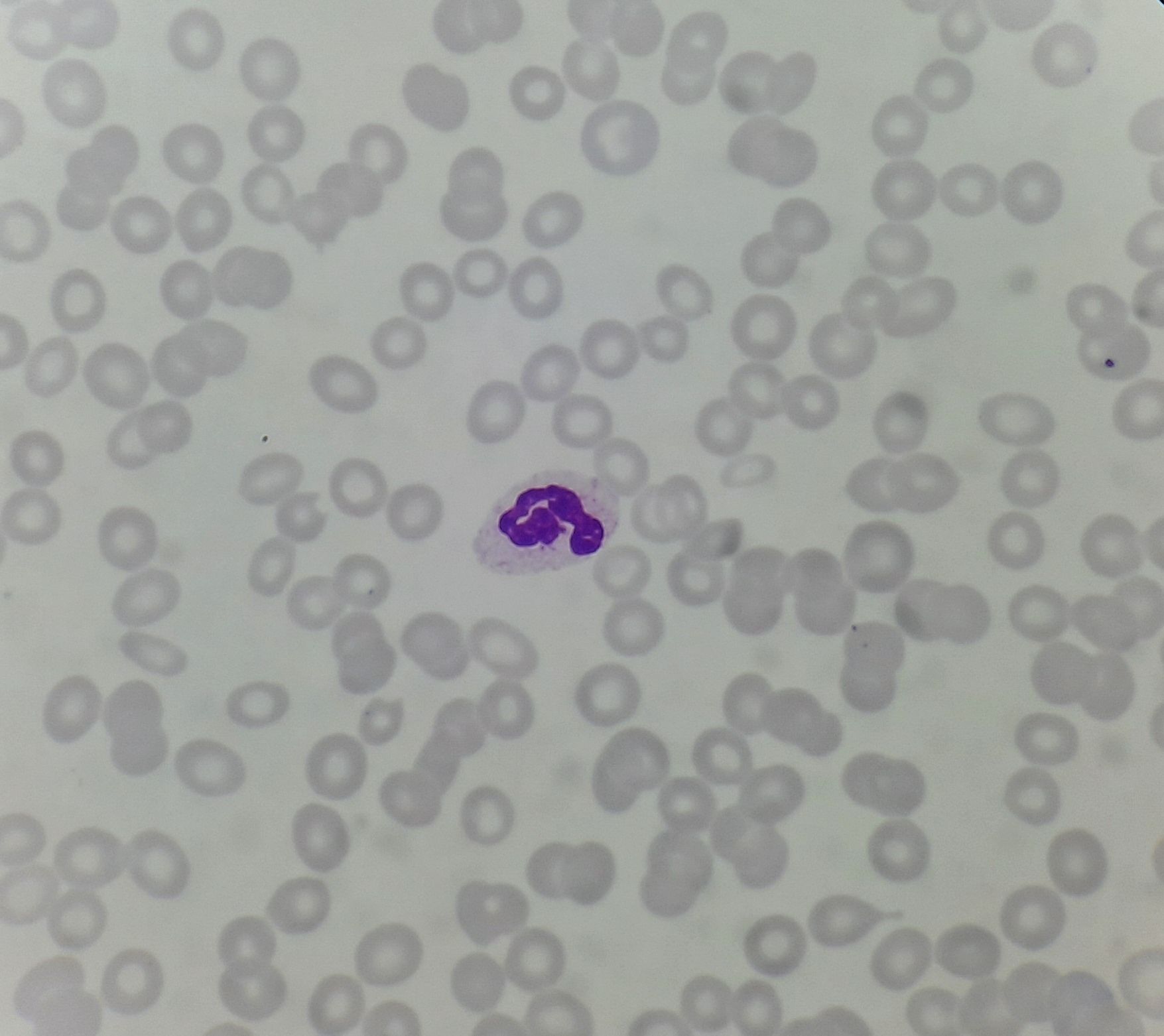
Figure 10.1.1- Immune thrombocytopenic purpura- Peripheral smear

Figure 10.1.2- Immune thrombocytopenic purpura- Bone marrow aspiration

Figure 10.1.3- Immune thrombocytopenic purpura- Bone marrow biopsy
Recent advances:
New medications in treatment of ITP:
- Anti CD38- Daratumumab, mezagitamab
- Anti CD40- IDEC-131, hu5c8, Letolizumab
- Immunoproteasome inhibitors: Bortezomib, KZR-616
- FcRn inhibitors- Efgartigimod, Rozanolixizumab
- Inhibitors of macrophage function: Hypersialytaed IVIg, Recombinant Fc multimers, Syk kinase inhibitors (Fostamatinib, sovleplenib), BTK inhibitors (Ibrutinib, rilzabrutinib)
Use of ATRA in ITP
All trans retinoic acid is know to exert immunomodulatory effect and promote thrombopoiesis. A study from China, looked at use of ATRA as upfront therapy in newly diagnosed patients with ITP. Compared to standard arm, patients in experimental arm, received ATRA (10mg BD for 12 weeks) in addition to high dose dexamethasone (40mg OD for 4 days). At 6 months, a significantly higher proportion of participants in the all-trans retinoic acid plus high-dose dexamethasone group (68%) than in the high-dose dexamethasonemonotherapy group (41%) had a sustained response.
https://doi.org/10.1016/S2352-3026(21)00240-4
Covid-19 vaccine Associated ITP- French study
Most of the vaccines used in prevention of SARS-CoV-2 infection increase the risk of development of de-novo and relapse of ITP. The French National Agency for the Safety of Medicine and Health Products set up an active monitoring for COVID-19 vaccine adverse drug reactions. The median time from vaccination to ITP onset was 11 days. 12% patients were only observed, 64% patients recovered with steroids and rest were treated with TPO agonists/ rituximab. Out of 7 patients who were rechallenged with vaccine 3 had relapse of ITP.
doi.org/10.1182/blood.2022015470
Rilzabrutinib, an Oral BTK Inhibitor, in Immune Thrombocytopenia
Rilzabrutinib, an oral, reversible covalent inhibitor of Bruton’s tyrosine kinase was tested in patients with immune thrombocytopenia. It acts mainly through 2 mechanisms: decreasing macrophage mediated platelet destruction and reducing production of pathogenic autoantibodies. Trial involved 60 patients of chronic ITP with median duration of disease 6.3 years. At a median of 167 days of treatment, 24 of 60 patients (40%) showed significant increment in platelet counts.
https://doi.org/10.1056/NEJMoa2110297
Treatment of immune thrombocytopenia with eltrombopag in patients who had and who had not received prior rituximab: post-hoc analysis of the EXTEND study
Eltrombopag is a TPO-RA approved in Europe for treatment of patients aged ≥1 year, with ITP lasting ≥6 months from diagnosis refractory to other treatments (e.g. corticosteroids). This subanalysis of EXTEND study assessed whether eltrombopag efficacy and/or safety were different among patients with and without prior rituximab treatment. Eltrombopag was effective and generally well tolerated, regardless of previous rituximab, splenectomy, or both.
https://doi.org/10.1111/bjh.17800
Avatrombopag in immune thrombocytopenia
Avatrombopag is a new oral TPO-RA. In the present retrospective observational study, adults with ITP who switched from eltrombopag or romiplostim to avatrombopag were evaluated. On avatrombopag, 41/44 patients (93%) achieved a platelet response (≥50 × 109/l) and 38/44 patients (86%) achieved a complete response (≥100 × 109/l).
https://doi.org/10.1111/bjh.18081
Should dexamethasone alone or in combination be the initial steroid for adult ITP: Still a relevant question
Four-day cycles of dexamethasone work faster in increasing platelet counts and appear to reduce the occurrence of severe adverse events. Therefore, it is probably a better option for patients with low platelet counts and bleeding diathesis; however, curative superiority, the initial reason to administer it, compared to PDN is not well demonstrated. Dexamethasone in combination with rituximab in first-line treatment produced higher response rates with better long-term results compared to high-dose dexamethasone alone and is a particularly good option in younger women.
https://doi.org/10.1111/bjh.18398
Antinuclear antibody titre in children with primary immune thrombocytopenia
This study examined the impact of antinuclear antibody (ANA) titre in children with primary immune thrombocytopenia (ITP) through a retrospective cohort study of 324 children at Peking Union Medical College Hospital. 39.2% had ANA titres of 1:160 or higher. Patients with higher ANA titres exhibited lower platelet counts at onset but had a higher rate of subsequent platelet count recovery. Moreover, patients with ANA titres of 1:160 or higher were more likely to develop autoimmune disease (AID), and the risk of AID increased with rising ANA titres.
https://doi.org/10.1111/bjh.18732
Long-term eltrombopag in children with chronic immune thrombocytopenia
This study provides long-term follow-up data on eltrombopag (ELT) use in children with chronic immune thrombocytopenia (cITP), spanning from September 2018 to June 2023. Among 65 patients, 29.23% discontinued ELT due to stable response, while 18.46% switched to other therapies due to loss of response (LOR) after a median of 19.13 months. Of those discontinuing ELT, 24.62% achieved a sustained response off-treatment, with a median platelet count of 107 × 10^9/L. Patients with LOR had lower platelet counts at ELT initiation and longer time to response compared to those who tapered and maintained a durable response. Overall, the study suggests that ELT remains effective and safe for long-term use in children with cITP.
https://doi.org/10.1111/bjh.19253
Dapsone for paediatric chronic immune thrombocytopenia
This study evaluated the efficacy of dapsone as a second-line agent in children with chronic immune thrombocytopenia (ITP). Among 45 children treated with dapsone, 37.8% showed an early response, and at least a partial response was observed in 64.4% of patients during a median follow-up of 50 months. These findings suggest that dapsone offers favorable initial response rates and sustained remission in pediatric chronic ITP, comparable to other available therapeutic agents.
https://doi.org/10.1111/bjh.19277
Autoimmune cytopenia and Kabuki syndrome
Kabuki syndrome (KS), caused by KMT2D and KDM6A variants, often presents with intellectual disability, characteristic facial features, and autoimmune cytopenias (AIC), including chronic immune thrombocytopenic purpura and Evans syndrome. In a study of 11 patients, pathogenic KMT2D variants were identified during the immunological evaluation for AIC, with a median of 8 associated KS manifestations per patient. Eight patients required second-line treatments like rituximab and mycophenolate mofetil, and long-term follow-up showed a continued need for treatment in most cases.
https://doi.org/10.1111/bjh.19387
Comparison of platelet transfusion effectiveness between Helicobacter pylori-positive and -negative immune thrombocytopenia
Immune thrombocytopenia (ITP) involves rapid platelet destruction and decreased platelet production, with Helicobacter pylori (H. pylori) infection playing a potential role in some Japanese patients. This study compared the efficacy of platelet transfusion in severe ITP patients with and without H. pylori infection. Results indicated that the median corrected count increment (CCI) at 24 hours post-transfusion was significantly higher in H. pylori-positive patients compared to H. pylori-negative patients (6463 vs. 754, p < 0.001). Multiple regression analyses confirmed that H. pylori infection was independently associated with better CCI-24, suggesting that platelet transfusion is more effective in H. pylori-positive ITP patients.
https://doi.org/10.1111/bjh.19413
Real-world use of thrombopoietin receptor agonists for the management of immune thrombocytopenia
In the TRAIT study, which retrospectively evaluated romiplostim and eltrombopag use in adult patients with immune thrombocytopenia (ITP) in the UK, 267 patients (median age 48 years) were included. Romiplostim was prescribed to 155 patients (58%), while 112 patients (42%) received eltrombopag as their first thrombopoietin receptor agonist (TPO-RA). Both treatments demonstrated efficacy, with 89% achieving a platelet count ≥30 × 10^9/L and 68% achieving ≥100 × 10^9/L with their initial TPO-RA. Treatment-free response (TFR), defined as maintaining a platelet count ≥30 × 10^9/L for 3 months after stopping treatment, was observed in 18% of patients.
https://doi.org/10.1111/bjh.19345
A Novel Anti-CD38 Monoclonal Antibody for Treating Immune Thrombocytopenia
In a phase 1–2 open-label study, CM313, an anti-CD38 monoclonal antibody, demonstrated safety and efficacy in adult patients with immune thrombocytopenia (ITP). Administered intravenously at 16 mg/kg weekly for 8 weeks, CM313 resulted in two consecutive platelet counts ≥50×10^9/L in 95% of patients, with a median response duration of 23 weeks. Common adverse events included infusion-related reactions (32%) and upper respiratory tract infections (32%). CD38-targeted therapy reduced CD56dimCD16+ natural killer cells, altered CD32b expression on monocytes, and decreased spleen macrophages in passive mouse models of ITP.
https://doi.org/10.1056/NEJMoa2400409
Switching from eltrombopag to hetrombopag in patients with primary immune thrombocytopenia
This post-hoc analysis of a phase III trial examined the effectiveness and safety of switching immune thrombocytopenia (ITP) patients from eltrombopag to hetrombopag. Among 63 patients who made the switch, response rates increased from 66.7% on eltrombopag to 88.9% on hetrombopag. Patients with lower platelet counts before switching also showed improved responses post-switch. Treatment-related adverse events were less frequent with hetrombopag, and no severe adverse events were reported. The findings suggest that hetrombopag is effective and well-tolerated, even in patients with limited response to eltrombopag, though further studies are needed to confirm these results.
https://doi.org/10.1007/s00277-024-05826-5
GPIbα CAAR T cells in treatment of immune thrombocytopenia
A breakthrough treatment for refractory and relapsed immune thrombocytopenia (ITP) may lie in chimeric autoantibody receptor (CAAR) T-cell therapy. This approach targets glycoprotein (GP) Ibα, a key autoantigen in ITP, which is associated with poor response to standard treatments. Researchers engineered T cells to express a GPIbα CAAR, which selectively lyses cells harboring anti-GPIbα B-cell receptors, addressing the root of the autoimmune response. In vitro and in vivo studies confirmed these CAAR T cells could eliminate GPIbα-specific B cells without affecting healthy B cells. This innovative therapy holds promise for ITP patients who fail conventional treatments.
https://doi.org/10.3324/haematol.2023.283874
Mental health in adult patients with primary immune thrombocytopenia
Patients with primary immune thrombocytopenia (ITP) experience a significantly higher risk of mental health issues, including depression, anxiety, and fatigue, compared to the general population. Analysis of nationwide registry data revealed a cumulative incidence of mental health events and increased use of psychotropic drugs, such as opioids, antidepressants, and benzodiazepines, in ITP patients, especially within the first year following diagnosis. These findings underscore the impact of ITP on mental health and highlight the need for addressing mental health concerns in the management of ITP.
https://doi.org/10.3324/haematol.2024.285364
Sovleplenib in adult patients with chronic primary immune thrombocytopenia
In the phase 3 ESLIM-01 trial, sovleplenib, a novel spleen tyrosine kinase (SYK) inhibitor, demonstrated significant efficacy and safety in treating chronic primary immune thrombocytopenia. Patients on sovleplenib achieved a 48% durable response rate, compared to none in the placebo group, with a median time to response of 8 days versus 30 days for placebo. While treatment-emergent adverse events were common, they were mostly mild to moderate, and sovleplenib had a tolerable safety profile.
https://doi.org/10.1016/S2352-3026(24)00139-X
Fostamatinib effectiveness and safety for immune thrombocytopenia
Fostamatinib, a Syk inhibitor for adult immune thrombocytopenia (ITP), was evaluated in 138 patients across 42 Spanish centers. The cohort had a median age of 66 years, with a median time since ITP diagnosis of 51 months and a median of four prior therapies. Seventy-nine percent of patients responded, with 53.6% achieving complete response, and monotherapy showed an 85.4% response rate. Adverse events were mostly mild, including diarrhea and hypertension. This real-world study confirmed fostamatinib’s effectiveness and safety in both primary and secondary ITP.
https://doi.org/10.1182/blood.2024024250
Diacerein in patients with ITP
Diacerein is a anthraquinone used to treat joint diseases such as osteoarthritis. This study compared the efficacy and safety of eltrombopag plus diacerein versus eltrombopag alone in patients with primary immune thrombocytopenia (ITP) who were unresponsive to 14 days of full-dose eltrombopag. The combination therapy significantly increased the overall response rate, with 44% of patients in the eltrombopag plus diacerein group responding by day 15, compared to only 13% in the monotherapy group, and this benefit was sustained at day 28. Eltrombopag plus diacerein also prolonged the duration of response and was well tolerated, with common adverse events including respiratory infections and gastrointestinal issues, offering a promising alternative for patients unresponsive to standard eltrombopag treatment.
https://doi.org/10.1182/blood.2024025067
Baricitinib in steroid-resistant or relapsed immune thrombocytopenia
A phase 2 trial evaluated baricitinib, a JAK inhibitor, in adults with refractory or relapsed immune thrombocytopenia (ITP), aiming to address immune dysfunction without inducing thrombocytopenia. Among 35 enrolled patients, 57% achieved a durable response at 6 months, with an initial response in 65.%. Responders showed a median time to response of 12 days and a median peak platelet count of 94 × 10⁹/L. After discontinuation, 44% maintained a response for a median of 20 weeks. Adverse events were reported in 31% of patients, including infections in 17%, and 5% discontinued due to adverse events. These results suggest baricitinib as a promising therapy for ITP, warranting further studies.
https://doi.org/10.1002/ajh.27433
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.