howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Inherited Thrombophilias
Introduction:
- Thrombophilias are disorders of haemostasis that appear to predispose to venous thrombosis
- Only 5-20% of patients with spontaneous venous thrombosis have identifiable genetic defect.
Patients with inherited thrombophilias have
- Venous thrombosis either spontaneously or of severity apparently out of proportion to any identifiable stimulus
- Recurrent events of thrombosis
- First event of thrombosis at young age
- High risk of thrombosis if there is an acquired risk factor
- Some of them (such as Factor V Leiden, Prothrombin G20210A) can have high risk arterial thrombosis
- Higher risk of pregnancy complications such as placental abruption, preeclampsia, early/late fetal loss and IUGR. All these are related to compromised blood flow.
Classification
- Reduced anticoagulant function
- Deficiency of antithrombin
- Deficiency of protein C
- Deficiency of protein S
- Increased procoagulant activity
- Factor V Leiden
- G20210 A prothrombin polymorphism
- Elevated levels of factor VIII, IX and XI
- Hyper homocysteinemia
- Homozygous C677T polymorphism in methyl tetrahydrofolatereductase
- Decreased fibrinolysis
- Dysfibrinogenemia
- Dysplasminogenemia
- Increased thrombin activable fibrinolysis inhibitor (TAFI)
Factor V Leiden
(Activated protein C resistance)
- Activated Protein C normally degrades Factor Va
- Factor V Leiden results from G1691A mutation, which results in substitution of glutamine for argentine at 506 position which is major site at which activated protein C acts.
- Hence Factor V Leiden is resistant to degradation by activated protein C. Because of this patient has high risk of thrombosis.
- APTT – Done with or without added APC
- Result are expressed in ratio (APC sensitivity ratio)
APTT of sample with added APC
APTT of sample without added APC
- Greater the resistance, lower the ratio
- Pretest dilution of test plasma with F V deficient plasma increases the specificity and sensitivity of test
- PCR test is available for detection of particular mutation
- Other resistant factor V
- FV Cambridge – 306 Arginine to threonine
- FV Hong Kong – 306 Arginine to glycine
Prothrombin G20210A mutation
- Single nucleotide change of guanine to adenine at position 20210 in the 3’ untranslated region of prothrombin gene is associated with increased plasma prothrombin levels, as this mutation augments translation and stability of prothrombin m-RNA.
- This results in increased synthesis of prothrombin.
- Increased thrombin levels lead to increased risk of thrombosis.
- Diagnosis can be confirmed by mutation testing by PCR
Antithrombin Deficiency
- Antithrombin III
- Synthesized in liver
- Glycoprotein member of family of serine protease inhibitors
- It inhibits activated clotting factors such as thrombin, IXa, Xa, XIa, XIIa and tissue factor bound F VIIa
- Rate of complex formation between antithrombin and activated clotting factors is markedly accelerated by heparin and by proteoglycans on the vascular endothelium
- More than 150 mutations have been identified leading to antithrombin deficiency
- 2 types of deficiencies
- Type I- Quantitative reduction of qualitatively normal antithrombin
- Type II- Defect in thrombin binding site (Reactive site)
- Type III- Heparin binding site defect. Hence activity along with heparin is low.
- Annual risk of venous thrombosis : 0.87 – 1.6%
- Antithrombin assay
- Antigen levels – Decreased in type I, Normal in type II
- Assays measuring heparin cofactor activity – Decreased in both
- Causes of acquired antithrombin deficiency
- Acute thrombosis
- Pregnancy, combined OC pills
- Severe liver disease
- L-Asparaginase
- DIC- Increased consumption
- Nephrotic syndrome Increased Loss
- IBD
- Heparin treatment
- Major surgery
- Recombinant and plasma derived antithrombin are available
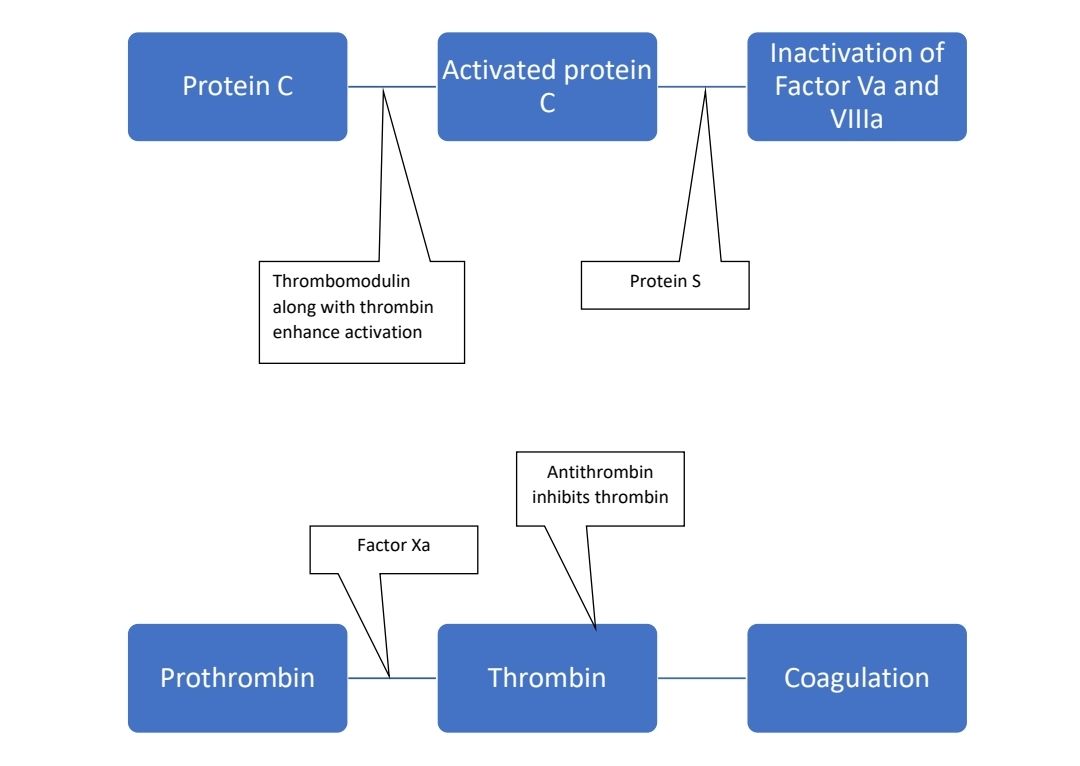
Protein C Deficiency
- Protein C
- Vitamin K dependent glycoprotein
- Synthesized in liver
- Circulated as 2 chain zymogen
- Activated by thrombin to activated protein C (APC)
- This activation process is enhanced to 1000 folds when thrombin is bound to thrombomodulin
- APC binds to proteins on the surface of activated cells and this complex then inactivates F Va and F VIII a (cleavage product of F Va acts as cofactor for factors and enhances degradation of F VIIIa)
- More than 150 mutations have been identified so far
- Types of protein C deficiency
- Type I - Decreased antigen level
- Type II - Decreased activity
- Protein C assays – Using specific activator Protac, which is derived from snake venom. Values less than 55% are considered deficient.
- Acquired causes of protein C deficiency
- Vitamin K antagonists (Oral anticoagulants)
- Vitamin K deficiency
- DIC
- Liver diseases
- Mutation in gamma glutamylcarboxylase gene
- Sepsis
- Purpurafulminans
- Progressive hemorrhagic skin necrosis in neonate with severe protein C deficiency at birth.
- Sometimes it is seen as post-viral event especially along with chicken pox
- Treatment: Activated protein C
- Warfarin induced skin necrosis
- Seen in heterozygous patients, when they are started on warfarin.
- As protein C is vitamin K dependent protein, warfarin decreases its levels rapidly. This leads to thrombosis in dermal blood vessels.
- Typically necrotic skin lesions are seen in extremities, breasts and trunk.
Protein S deficiency
- Protein S
- Vitamin -K dependent glycoprotein
- Synthesized in liver, endothelial cells, megakaryocytes and testicular Leidig cells
- 60% is circulated bound to beta- chain of C4 binding protein & is inactive
- 40% is free form which is active
- Free proteins increase the affinity of APC for negatively charged phospholipid surfaces on platelets or the endothelium, enhancing complex formation of APC with F Va & F VIII a
- More than 200 mutations have been identified
- Types of Protein S deficiency
- Type I- Quantitative defect caused by genetic variation (Both total and free from levels are reduced)
- Type II- Qualitative defect
- Type III- Total proteins level is normal but there is reduction in free proteins antigens
- Protein S assay – By ELISA or RIA- Precipitation with polyethylene glycol followed by centrifugation
- Functional assays are based on APC cofactor activity
- Causes of acquired deficiency
- Pregnancy
- Estrogen- OCP, HRT
- Vitamin -K antagonists
- DIC
- Liver damage
- Inflammatory conditions
- Acute thromboembolism
- Lupus anticoagulants
- L-asparginase
Dysfibrinogenemia
- Associated with increased risk of thrombosis in pregnancy
- Defective binding of thrombin to abnormal fibrin which leads to
- Decreased activation of Protein C by thrombin
- Elevated thrombin levels
- Defect in binding of tissue plasminogen activator to fibrinogen
Elevated factor VIII levels
- Levels more than 1500 U /L are associated with high risk of thrombosis
- Other causes of increased factor VIII levels include
- Increased age
- Obesity
- Pregnancy
- Surgery
- Inflammation
- Liver diseases
- Hyperthyroidism
- Diabetes mellitus
Increased Homocysteine levels
- Homocysteine is intermediate in metabolism of sulphur containing amino acids such as methionine and cysteine
- Remethylation of homocysteine to form methionine requires vitamin B12 depended enzyme such as Cystathione beta synthase and Methylenetetrahydrofoltereductase.
- Hence homocysteine levels are elevated in cases of
- Deficiency of Cystathione betasynthase and Methylene tetrahydrofolte reductase
- Vitamin B12 deficiency
- Other conditions such as renal failure, hypothyroidism, smoking, excess coffee, inflammatory bowel disease, psoriasis, rheumatoid arthritis
- How increased homocysteine causes thrombosis?
- Deleterious effect on endothelium
- Enhanced smooth muscle proliferation
- Induction of tissue factor by monocytes
- Reduced cleavage of Factor V by APC
- Suppression of heparin sulphate synthesis
- Down regulation of thrombomodulin
- Congenital hyperhomocysteinemia presents with
- Severe mental retardation
- Seizures
- Skeletal deformities
- Ectopialentis
- Premature Vascular disease with severe atherosclerosis
- Venous thromboembolism
- Stroke and arterial thrombosis
- Investigations
- S. Homocysteine levels- >100 micromol/L
- MTHFR mutation study
- High doses of vitamin B12, folic acid and Vitamin B6 should in added to treatment.
Other rare inherited thrombophilias include
- Increased levels of F IX
- Increased levels of F XI
- Increased fibrinogen level
- Increased plasminogen level
- Decreased plasminogen activator inhibitor 1
- Increased TAFI
- Increased Protein C inhibitors
- Mutations in thrombomodulin
- Mutations of endothelial protein C receptor
Thrombophilia screen
It includes
- Coagulation screen – APTT, PT, TT
- Liver function tests
- Renal function tests including electrolytes
- Complete hemogram- For polycythemia vera and other MPNs
- Urine routine- For nephrotic syndrome
- APLA work up
- Anti-cardiolipinIgG and IgM antibodies
- Anti Beta 2 glycoprotein I- IgG and IgM antibodies
- DRVVT
- Antithrombin activity – Heparin cofactor assay
- Protein C – Chromogenic assay
- Protein S – Immunoreactive assay
- Modified APC / SR ratio
- Factor V Leiden – PCR
- Prothrombin G20210 – PCR
- Activated protein C resistance (diluting patient plasma with factor V-deficient plasma)
- Prothrombin G20210A mutation testing by polymerase chain reaction
- Fasting total plasma homocystein level
- Factor VII activity
- MPN mutation testing
- PNH work up
Indications for thrombophilia screen
- Unprovoked venous thromboembolism in young (<40 years)
- Venous thromboembolism in unusual sites
- Recurrent venous thromboembolism
- Venous thromboembolism in the setting of a strongly family history of venous thromboembolism
- Unexplained recurrent pregnancy loss
- Family members of venous thromboembolism patients with known inherited hypercoagulable states
Optimal timing for thrombophilia screen
- Not advised during period of acute thrombosis and active treatment, as
- In acute thrombosis state, levels of antithrombin, protein C and protein S are low
- Heparin decreases activity of antithrombin
- Warfarin decreases levels of protein C and protein S
- It should be done at the time when patient is off anticoagulation for several weeks without recent thrombosis
- If anticoagulation cannot be stopped
- Do all tests except protein C and S when patient is receiving warfarin
- Then switch to LMWH for 2 weeks and then test protein C and S.
- Later restart warfarin in previous dosage
Therapeutic implications
- Very less clinical trial data
- Hence better to give lifelong anticoagulation, if thrombophilia positive patient had developed unprovoked VTE.
- Initiation and intensity of anticoagulation is same as any other VTE case
- Close family members must be tested for genetic abnormality. This helps in
- Avoiding environmental risk factors such as OCPs, smoking, obesity etc
- Providing thromboprophylaxis during high risk situations such as surgeries or post partum period
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.