howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Introduction to Mature T and NK Cell Neoplasms
Compared to B-NHL, Peripheral T cell Lymphomas:
- Present in more advanced disease
- Poorer performance status
- Frequent B symptoms
- Frequent paraneolastic features such as eosinophilia, HLH etc
- Higher incidence of autoimmune phenomenon
PTCL-NOS, includes those cases that do not belong to any of the better defined entities
Epidemiology:
- Account for 6% of all NHL
- Usually seen in adults
- Common in men
- Median age- 61 years
- PTCL-NOS accounts for 50% of all PTCLs.
Etiology:
- Risk factors include HTLV1 and EBV infections
Clinical Features:
- Bulky lymphadenopathy
- B symptoms
- Common extranodal involvement
- Sometimes- HLH
Investigations:
- Lymph node biopsy- Features seen in PTCL- NOS are
- Diffuse infiltrates of tumor cells with effacement of normal nodal architecture
- Very broad cytological spectrum mainly medium to large sized cells
- Nuclei are irregular, pleomorphic and may be hyperchromatic with prominent nucleoli
- Clear cells and RS like cells often present
- High endothelial venules increased
- An inflammatory, polymorphous background seen.
- Immunohistochemistry:
- T-Cell associated antigens are positive, but there can be loss of one or more antigens (Especially CD7)
- Positive- CD45, CD43, CD3
- 80% are CD4 + / CD8 –
- 20% are CD4 – / CD8+
- Molecular studies: Clonal rearrangement of the T cell receptor gene
- Cytogenetics:
- Loss of 9p, 5q or 12p.
- Gains of 7q, 17q.
- Complex cytogenetic abnormalities
- t (5:9)- Fusion of IL2 inducible T cell kinase with spleen tyrosine kinase
- Hemogram- Eosinophilia
- LDH- Increased
Prognosis:
- Generally Poor
- Overall 5 year survival- 30%
- T Cell specific International Prognostic Index score: One point given to each of these:
- Age >60 years
- Raised S. LDH levels
- PS- 2-4
- Stage III or IV
- Extranodal involvement > 1 site
Score | Risk | 5 year survival |
0-1 | Low | 62% |
2 | Low-intermediate | 53% |
3 | High-intermediate | 33% |
4-5 | High | 18% |
- Other poor prognostic markers include:
- High Ki 67 expression
- Extranodal disease
- Expression of cytotoxic molecules- TIA-1, Granzyme-B, nm23-H1 protein
- TP53 gene abnormality
- Chemokine receptor expression- Ex: CCR4
- B Symptoms
- Bulky disease- >10cm
- Raised CRP levels
- Platelet count <1,50,000/cmm
- Loss of 5q, 10q and 12q carry better prognosis
Pretreatment Work-up for PTCL NOS:
- History
- B-Symptoms
- Examination
- LN:
- Spleen:
- Full skin exam:
- WHO P. S.
- BSA
- IHC
- BMA and Bx
- CT (CAP)/ PET
- Stage
- Hemoglobin
- TLC, DLC
- Platelet count
- LFT - Bili- T/D SGPT: SGOT: Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid:
- LDH
- Beta2 microglobulin
- HIV:
- HBsAg:
- HCV:
- HTLV 1 Serology (In high risk population)
- UPT
- IPI score
- ECHO (If anthracyclines planned) LVEF- %
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
Treatment Plan for PTCL-NOS:
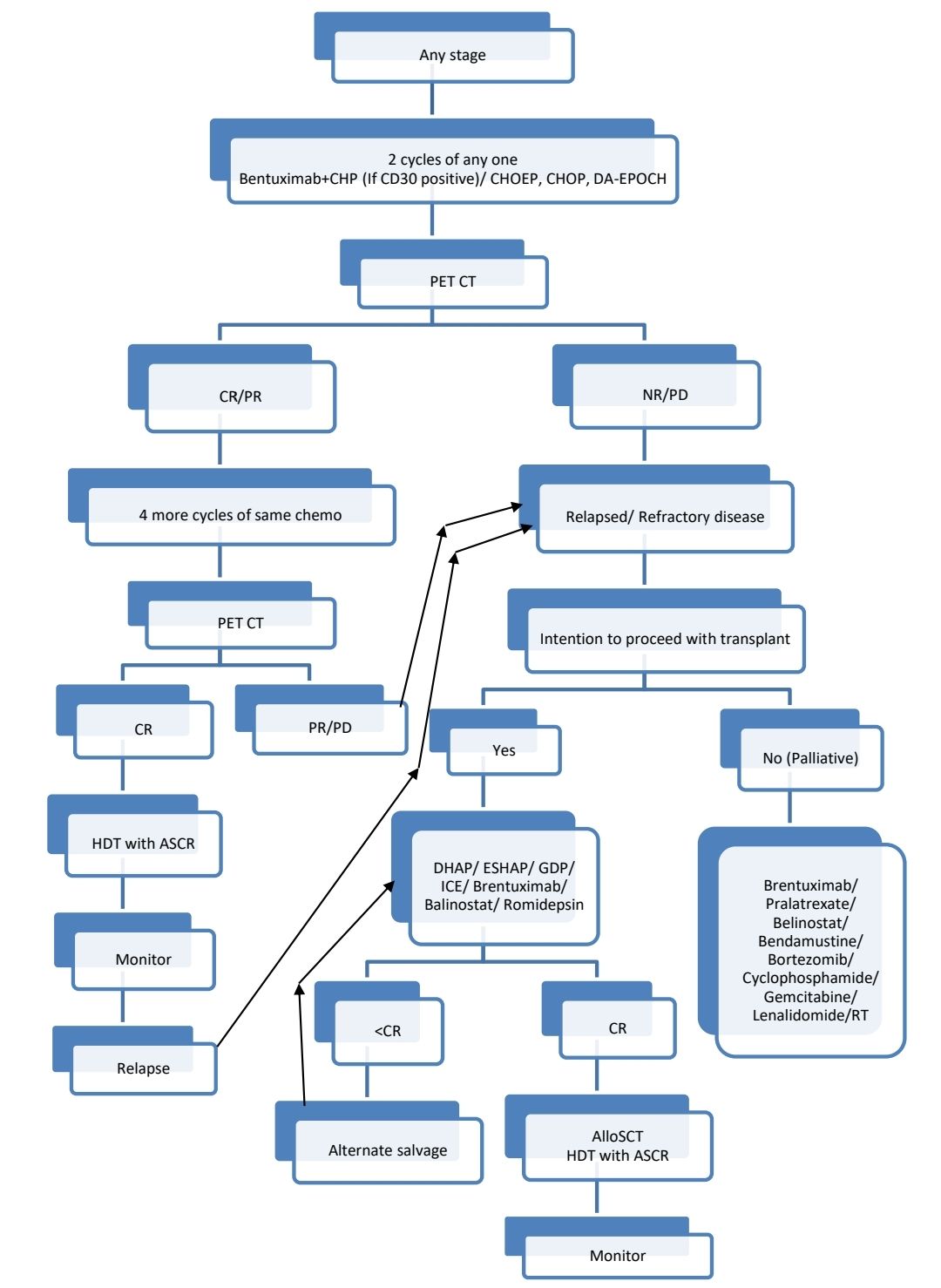
Indications for CNS prophylaxis
- High IPI score
- High LDH
- Involvement of extranodal site
Other Treatment Options:Targeted therapies
- Sipilizumab:
- Anti CD2 antibody
- 0.4-1.2mg/kg- biweekly- for total of 8 doses
- It triggers T cell apoptosis
- Anti-CD3 antibodies
- Muromonab: Causes high INF alpha release with severe cytokine syndrome, hence not used
- Visilizumab: Engages CD3, but does not cause release of TNF alpha
- Zanolimumab:
- Anti CD4 antibody
- Has cytotoxic and antiproliferative activity
- Given biweekly for 6 weeks
- Dose: early stage: 560mg, Late stage: 980mg
- Alemtuzumab- Anti CD52 antibody
- Brentuximab: Anti CD30 antibody
- Daclizumab- Anti CD 25 antibody
- Anti CD 194 antibody
Recent advances:
Allogeneic hematopoietic stem cell transplantation for NK/T-cell lymphoma: an international collaborative analysis
This retrospective analysis examined the role of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in patients with relapsed/refractory natural killer/T-cell lymphomas (NKTCL). The study included 135 patients who underwent allo-HSCT between 2010 and 2020. The three-year progression-free survival (PFS) and overall survival (OS) rates were 48.6% and 55.6%, respectively. Shorter time interval between diagnosis and allo-HSCT and transplantation not in complete or partial response were associated with reduced PFS. Pre-transplant treatment with programmed cell death protein 1 (PD-1/PD-L1) inhibitors did not affect graft-versus-host disease or survival.
https://doi.org/10.1038/s41375-023-01924-x
CHOEP plus lenalidomide as initial therapy for patients with stage II–IV peripheral T-cell lymphoma
In this phase II study, a novel induction strategy for nodal-based peripheral T-cell lymphoma (PTCL) was evaluated using lenalidomide in combination with CHOEP chemotherapy. Patients received standard doses of CHOEP along with 10 mg of lenalidomide on days 1–10 of a 21-day cycle for six cycles. Among 39 evaluated patients, the objective response rate after six cycles was 69%, with complete response in 49% and partial response in 21%. However, hematologic toxicity, including febrile neutropenia, was a significant issue, leading to treatment discontinuation in some cases. Despite these challenges, the estimated 2-year progression-free and overall survival rates were 55% and 78%, respectively.
https://doi.org/10.1111/bjh.18885
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.