howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Iron Deficiency Anemia
Introduction:
- Iron deficiency anemia is the most common form of anemia throughout the world.
- In America during 1870 to 1920, it was called as “chlorosis”. Iron salts were given for this condition even though exact nature of disease had not been identified
Iron requirement
- Average requirement for an adult is about 1mg/day
- This is to replace iron loss through sweat, urine, bile, desquamation etc.
- 5 -10 % of ingested iron is absorbed, so diet should contain about 15mg of iron.
- During monthly menstruation 41-46ml of blood is lost. This amounts to loss of 20-30mg of iron, so menstruating females need 2mg of iron per day.
- During pregnancy iron requirement is 3.4 mg/day (Total – 1000mg throughout 9 months).
Iron kinetics
- Absorption
- Absorption of iron occurs mainly in duodenum
- Out of 15mg of average daily intake 3mg is absorbed (1.5mg with vegetarian diet)
- Ferric complexes of non heme iron are reduced first to ferrous form
- Gastric hydrochloric acid, dietary lactic acid, ascorbic acid keep iron in dissolved form and help in their absorption.
- Certain sugars, monoacids and amines prevent polymerization and precipitation of iron complexes and aid in absorption.
- Carbonates, oxalates, phosphates, phytates, tannates combine with iron to form insoluble complexes which cannot be absorbed
- Heme iron is readily absorbed (Direct assimilation by mucosal cells)
Molecular aspects of iron absorption
1
Non-heme iron is released from food as Fe3+ and is reduced to Fe2+ by Duodenalcytochrome b
↓
Fe2+ is transported across brush border membrane of enterocyte by DMT (Divalent membrane transporter)- 1
↓
Enters the labile pool
↓
Transported across the serosal membrane is by ferroportin 1
↓
Conversion of Fe2+ to Fe3+ is done by Hephaestin- a copper containing ferro-oxidase
↓
Taken up by transferrin as Fe3+
2
Heme iron binds to heme receptors on brush border
↓
Released intracellularly by hemeoxygenase
↓
Enters the labile pool
Hepcidin
- Product of HAMP gene
- Small peptide containing 20-25 amino acids
- Expressed in liver
- Down regulated when iron stores are reduced
- Up-regulated when iron stores are increased and in inflammatory states
- It binds to ferroportin and accelerates its destruction. So it decreases
- Iron absorption in intestine
- Iron transport across placenta
- Iron release from macrophages
Transport
Once in the mucosal cell, iron combines with apoferritin to form ferritin
Or
It crosses into plasma and binds to transferrin
↓
Transferrin is equally distributed between plasma and extravascular space
It exchanges iron with all cells of body which bear transferrin receptor
(Synthesis of this receptor is controlled via an iron dependent negative feedback in cells)
↓
Iron enters the cell in an energy and temperature dependent process called as ropheocytosis
↓
Transferrin receptor complexes on the cell membrane and the membrane invaginates, forming a pit with complex inside. Endosome is thus formed.
↓
This endosome fuses with acidic vesicles
At pH < 5.5 iron is released form transferrin and made available for cellular use.
↓
Endosome is transported back to the surface of cell and consequently transferrin is released into plasma.
Breakdown of hemoglobin bound iron in macrophages
- Macrophages phagocytose senescent RBCs
- Heme is broken down by heme-oxygenase to release iron
- Part of it binds to Ferritin and part is released into plasma via ferroportin which binds to transferrin
- 5 -10 % of hemoglobin from senescent RBCs is released intravascularly, which is bound to haptoglobulin. This is removed by macrophages.
Iron stores:
- Iron is stored in the form of ferritin in hepatocytes and hemosiderin in reticulo endothelial system
- Amount of stored iron
- Newborn – 80mg / kg
- 6 months to 2 years – Nil
- Childhood – 5mg / kg
- 15 - 30 years – 10 – 12 mg / kg (Low in women)
- It takes 7 years or more for a man to deplete body iron stores solely due to lack of dietary intake.
Cellular iron homeostasis
Low cellular iron levels
↓
IRP (Iron response protein) 1 binds to iron response element of mRNAs which code for transferrin receptor and ferritin.
(When iron is abundant IRP-1 contains iron sulphur cluster which acts as aconitase and has no affinity for iron response element of mRNA, and
Hence it undergoes rapid proteolysis)
↓
This binding protects transferrin receptor and ferritin mRNA from cytoplasmic degradation
↓
Increased synthesis of transferrin receptor and ferritin
Other mRNAs having iron responsive elements (IRE)
- Erythroid and ALA synthase mRNA
- Mitochondrial aconitase mRNA (It interconverts citrate and isocitrate)
- DMT 1
- Ferroportin 1 mRNA
Transferrin:
- It is β globulin synthesized in liver
- Single chain polypeptide, present in plasma
- MW – 79, 500
- Concentration-1.8–2.6g/L
- Synthesized in liver (synthesis in inversely related to iron stores)
- 2 Ferric atoms can bind to each molecule
- Function – Transport of iron
- Binding sites (C & N terminus) contain 3 tyrosine, 2 histidine& 1 arginine group.
- Plasma half life of transferrin in 5 days
- Each gram of transferrin can bind to 1.25 microgram of iron.
Transferrin receptor:
- M W – 18500
- It is a trans-membrane protein with 2 subunits, each bind to 1 molecule of transferrin
- Identified with anti CD71 antibodies.
- Transferrin has another receptor called TFR2, which acts as homeostatic iron sensor, regulating hepcidin synthesis in response to diferrictransferrin concentration.
Ferritin
- It is a major storage form of iron found in bone marrow, spleen and liver
- It is colorless and not visible microscopically
- It gives a faint bluish tint to tissues, when stained with ferrocyanide method
- It has outer coat of protein known as apoferritin and core contains iron.
- Molecular weight is 450000
- Apoferritin contains 24 subunits (There are 2 immunologically distinct types of subunits – H & L, which are coded by genes on chromosome 11 and 19 respectively)
- Each molecule of ferritin contains up to 4500 iron molecules.
- Iron in ferritin is present as hydrous ferric oxide phosphate
- Internal cavity of ferritin molecule communicates with exterior via 6 channels, through which iron enters and leaves.
- Variousisoferritins can be separated by electrophoresis and difference in them is with respect to content of H and L submits
- Heart and Red cell ferritin are rich in H (Acidic)
- Liver, spleen and serum ferritin are rich in L (Basic)
- Mitochondrial ferritinis a novel H type ferritin
- Entire ferritin molecule is degraded within lysosomes to release iron
- Amount of circulating ferritin parallels concentration of storage iron in body.(1 ng / ml indicates 8mg of stored iron)
- Less than 12ng / mg do not correlate with storage iron, because all stores are depleted at that level
- Normal level – 15-300 ng / ml
Hemosiderin:
- It is a water insoluble heterogeneous iron protein aggregate
- 50% of its weight is composed of iron.
- In unstained tissue specimens it appears as irregular golden brown granules and stains blue with Prussian blue iron stain.
- With aging of ferritin, apoferritin denatures and hemosiderin is formed.
Enzymes containing iron:
- They are responsible for electron transport pathway & production of ATP (Cytochromes a, b, c, Succinatedehydrogenase, Cytochromeoxidase)
- Cytochrome P 450 is involved in hydroxylation & detoxification of drugs
- Cyclooxygenase is involved in prostaglandin synthesis
- Catalase and Lactoperoxidase- Involved in breakdown of H2 O2
- Trytophanpyrrolase- Involved in oxidation of tryptophan to formylkynurenine
- Iron sulphur proteins- Xanthineoxidase, NADH2, Aconitase
- Ribonucleotidereductase – key enzyme in DNA Synthesis
Epidemiology of Iron deficiency:
- It is the most common cause of anemia
- >30% of population is affected by iron deficiency anemia
- More common in women in child bearing age
Etiology:
- Increased requirement
- Post natal growth spurt (6 -24 months)
- Adolescent growth spurt.
- Menstruating women
- Pregnancy and lactation.
- Inadequate ingestion in diet-
- Diet based on milk and deficient in meat
- Increased loss (0.5mg iron is lost per ml of blood loss. 6-8 ml blood loss/day is significant)
- Chronic GI Bleeding (Most common cause in postmenopausal women and adult men)
- Hiatus hernia
- Ulcerative disease of stomach/ intestines
- Gastritis
- Carcinoma- Stomach, colon- their initial presentation is often iron deficiency anemia.
- Hemorrhoids
- Arteriovenous malformations
- Angiodysplasia
- Esophageal varices
- Menetrier disease
- Celiac disease
- Meckel'sdiverticulum
- Bleeding disorder especially due to platelet dysfunction
- Hook worm infestation
- Menorrhagia
- Chronic Hemoptysis / hematuria
- Intravascular hemolysis with hemoglobinuria and hemosiderinuria. Ex: PNH, March hemoglobinuria
- Idiopathic pulmonary siderosis
- Nosocomial/ iatrogenic- Repeated blood sampling
- Lasthenie de Ferjol syndrome- Anemia due to self inflicted bleeding
- Chronic GI Bleeding (Most common cause in postmenopausal women and adult men)
- Malabsorption
- Celiac disease
- Gastrectomy / gastroenterostomy
- Atrophic gastritis
- H. Pylori infection
- Genetic: Transferrinpleomorphism, platelet collagen receptor pleomorphism
- Iron refractory iron deficiency anemia-
- Mutation of membrane serine protease- tmmprss6
- Normally this protease inhibits hepcidin production
- Hence there is increased concentration of hepcidin
- These patients do not respond to oral iron and have incomplete response to IV iron
- Functional iron deficiency (Inadequate supply of iron to bone marrow in presence of adequate storage iron. Low reticulocyte hemoglobin is an early indicator for functional iron deficiency)
- Chronic kidney disease
- Chronic inflammation
Clinical Features:
- Asymptomatic
- Anemia presenting as fatigue, tachycardia, palpitation, pounding in ears, headache, light headedness, angina, irritability
- Non-hematological manifestations may be present in absence of anemia. This occurs due to depletion of essential iron containing enzymes throughout the body (Epithelial cells need higher amount of iron)
- Glossitis - Atrophy of papillae – Pale, smooth, shiny, glazed tongue.
- Angular stomatitis – Redness, soreness and cracking.
- Platynychia followed by koilonychea – Nails first become thin, lusterless, brittle and show longitudinal ridging and flattening. Later they become spoon shaped.
- Blue sclera
- Hair loss
- Pica
- Phagophagia – Ice eating
- Geophagia – Earth eating
- Amylophagia – starch eating.
- Eating salt, cardboard, hair
- In children- irritability, loss of memory, difficulty in learning
- In Infants: Poor attention time, poor response to sensory stimulus, retarded behavior and developmental achievements
- Hyperactivity syndromes: Restless leg syndrome (Nocturnal muscle spasm), Tourette syndrome, attention deficit hyperactivity disorder
- Cognitive and intellectual impairment, which are irreversible
- Plummer Vinson syndrome (Paterson Kelly syndrome): It consists of traid of
- Microcytichypochromic anemia
- Atrophic glossitis.
- Esophageal post cricoid webs leading to dysphagia (If longstanding it can lead to pharyngeal carcinoma)
Investigations:
- Hemogram
- Microcytic hypochromic anemia
- Moderate to marked anisocytosis and poikilocytosis
- Abnormal shapes such as elliptical forms, pencil shaped cells, and target cells etc. are seen
- MCV – Decreased to 55 – 74fl
- MCHC – Decreased to 22 – 31gl/dl
- MCH – Decreased to 14 – 26pg
- RBC Count- Normal/ Decreased
- WBC Count- Normal.Eosinophilia is seen if there is worm infestation
- Platelet count- Increased in 50-70% of patients. Decreased if there associated ITP.
- Red cell distribution width (RDW) - more than 19. It also helps in differentiating iron deficiency anemia from thalassemia
- Reticulocyte count - Normal or slightly increased. In relation to anemia count it is decreased i.e. RPI is less than 2.
- Total iron binding capacity – Increased to more than 50 %
- It is the capacity of transferrin to bind to iron.
- Normal- 44-80micromol/L
- Serum iron levels :
- Initially it is normal, but later decreases to less than 50 microg/lit.
- Normal – 80- 180 microg/ lit
- Levels are highest in the morning (7-10am)
- Levels are decreased during menstruation, inflammatory states, malignancies
- It shows very short fluctuations, so it does not reflect iron supply over long time
- Plasma transferrin saturation level – decreased to less than 15%
- It is the percentage of total iron binding protein to which iron is attached.
- Transferrin saturation (T. Sat) = (Serum iron / Total iron binding capacity) x 100
- Normal – 33%
- Serumferritin level –
- Normal – 15 – 300mg/L
- Less than 15 mg/L is diagnostic of iron deficiency
- 15-100 mg/L indicates probable iron deficiency.
- It is the single most sensitive tool for evaluating iron status in community
- It reflects iron stores in the body. Its levels are not affected by recent intake of iron.
- It is earliest to fall in iron deficiency anemia
- Low levels are observed with hypothyroidism and ascorbate deficiency
- Low levels indicate iron deficiency, but high levels do not rule out iron deficiency
- In following conditions there is nonspecific rise in ferritin levels, so ferritin level assessment is not useful in following conditions
- Liver damage, splenic or bone marrow infarction (Release of tissue ferritin into circulation)
- Inflammatory states (Ferritin is an acute phase reactant protein)- concurrent CRP levels may be helpful in interpreting higher ferritin values
- Tumors such as acute leukemia and Hodgkin’s lymphoma.
- Hereditaryhyperferritinemia with cataract
- Hemochromatosis
- Goucher's disease
- Chronic renal disease
- Alcoholism
- HLH
- Metabolic syndrome
- Ineffective erythropoiesis- MDS, thalassemia etc
- Erythrocyte Ferritin level: Decreased
- Increased in thalassemia, sideroblastic anemia
- It is not influenced by inflammation
- Bone marrow examination
- Moderate erythroid hyperplasia
- Micronormoblastic erythropoiesis- Nucleus of normoblasts is pyknotic even when cytoplasm is polychromatic (This is because cytoplasmic maturation lags behind the nuclear maturation due to iron deficiency).
- Cytoplasm of normoblasts is scanty, stains irregularly and has ragged border.
- Erythroid nuclear abnormalities resembling dyserythropoietic anemia (budding, karyorrhexis, nuclear fragmentation and multinuclearity) are sometimes present
- Granulocytic precursors and megakaryocytes are normal
- Disappearance of stainable iron (Pearl’s stain)
- Erythrocyte protoporphyrin level
- Elevated to more than 100-600 microg/dL( Normal – 20 – 40 microg/dL)
- In case of insufficient iron, protoporphyrin accumulates in the cell (Detected as Zinc protoporphyrin as Zinc gets incorporated in protophorphyrin ring in place of iron)
- Its level in thalassemia is not raised, so it can be used to differentiate microcytic hypochromic anemias.
- It is raised even in lead poisoning, as lead inhibits ferrochelatase, an enzyme needed to incorporate iron into protoporphyrin ring.
- Its level is raised also in case sideroblastic anemia, erythropoieticporphyria and in condition with elevated erythropoiesis
- It is more sensitive in detecting iron deficiency with chronic inflammation, as serum ferritin is falsely increased in this condition.
- ZPP persists throughout the life span of RBC, hence diagnosis of iron deficiency can be made even after starting iron therapy
- Measured in a simple instrument called hematofluorometer (RBCs with excessive protoporphyrin fluorescence in UV light)
- Serum levels of transferrin protein :
- Increased to 13.91 ± 4.63 mg / L
- Normal - 5.36. ± 0.82 mg / L
- Assay is done by ELISA using monoclonal antibodies.
- It is very useful in differentiating iron deficiency anemia from anemia of chronic diseases.
- It is directly related to extent of erythroid activity and is inversely related to iron supply to cells.
- Serum levels of soluble transferrin receptor protein (STfR)
- This receptor synthesis is greatly increased when cells lack iron
- Majority is derived from erythroid marrow
- It is directly related to extent of erythroid activity and is inversely related to iron supply to cells
- Normal- 5.36mg/L
- In iron deficiency increased to >13mg/L (It may also be increased in case of rheumatoid arthritis, thalassemia trait, AIHA, Hereditary spherocytosis, HbH disease, sickle cell anemia)
- Decreased in case of Chronic renal failure, aplastic anemia
- Reticulocyte hemoglobin content- Decreased. It is the first test which indicates iron deficient erythropoiesis.
- S. Hepcidin level- Decreased
- Upper and lower GI scopies-
- To rule out GI malignancies
- These are must for all men and post menopausal women with iron deficiency anemia
- Urine for blood
- CT Colography- If colonoscopy is not possible
- Tests for celiac disease
- Serology: Tissue transglutaminase antibody or endomycial antibody
- Duodenal biopsy: To be done if serology is positive
- Stool for occult blood- As bleeding may be intermittent, this test is of no use.
- 51 Cr Sodium chromate tagged RBC scan- To know the site and quantity of blood loss
- Percutaneous retrograde angiography of celiac/ mesenteric arteriescan detect site of blood loss if loss is >0.5ml/min
- Scintigraphy (Tc)- To identify bleeding Meckel'sdiverticulum
- H. Pylori test- Especially in iron deficiency patients who are not responding to therapy
- Iron stain of sputum- To be done if intrapulmonary hemorrhage is suspected
Criteria for Diagnosis:
- Microcytic hypochromic anemia with Ferritin- Less than 15 microgm/L Or Transferrin Saturation- Less than 15%
- Therapeutic trial of oral iron for 3 weeks is less invasive and aids diagnosis in some doubtful cases. This strategy is useful if various techniques for diagnosis of iron deficiency do not exist. Reticulocytosis occurs in 1 week. Hemoglobin increases by 2gm/dL by 3 weeks.
- Reduced BM iron stores (Earlier it was considered as gold standard. However, some studies have results contradictory to this notion)
Differential Diagnosis:
- Refer: Approach to diagnosis- Anemia- Microcytic hypochromic anemia
Pretreatment Work-up:
- History
- Examination
- Hemoglobin
- TLC, DLC
- Platelet count
- Peripheral smear
- Reticulocyte count
- Ferritin
- Iron study: S. Iron: TIBC: T. Sat:
- Stool for occult blood
- Endoscopies(In men and postmenapausal women)
- Upper:
- Lower:
- LFT: Bili- T/D SGPT: SGOT:Albumin: Globulin:
- Creatinine
- LDH
Treatment:
- Treatment of underlying cause: Always try to identify the cause once iron deficiency is treated. This includes doing upper GI scopy and colonoscopy in postmenapausal women and all men.
- Oral iron therapy :
- Dose- 100-200mg of elemental iron per day.
- Ferrous sulphate - 200mg - TID or Ferrous Fumarate 200mg OD (T. Livogen contains Ferrous Fumarate 152mg= 50mg elemental iron and Folic acid- 1.5 mg, hence given twice a day)
- Prescribe by brand names, as pharmacist may give enteric coated tablet
- Only ferrous salts are better absorbed
- Tablet should be taken 1hr prior to food with acidic juices or along with Vitamin C.
- Side effects include nausea, epigastric pain, diarrhea and constipation. (These symptoms may be ameliorated by starting at lower doses and then slowly increasing the dose, reducing the dose to minimal effective dose, and by taking iron with food)
- Enteric coated and sustained release tablets are useless, as there is very little absorption of iron beyond duodenum
- Causes for failure of iron therapy
- Patient not taking therapy regularly
- Continued hemorrhage
- Continued malabsorption
- Associated Inflammatory illness, malignancy, hepatic disease or renal disease
- H. Pylori infection
- Large amounts of black tea
- Enteric coated tablets
- Inadequate dose
- Other options of oral iron
- Carbonyl iron
- Ferrous gluconate
- Ferrous succinate
- Ferric aspartate
- Polysaccharide ferrihydrite complex
- Parenteral iron therapy:
- Indications for parenteral therapy.
- Intolerance to oral iron – Severe nausea, vomiting, abdominal pain, diarrhea, constipation.
- Refractoriness to oral iron – Non compliance / persistent hemorrhage.
- GI disease whose symptoms are aggravated by oral iron like peptic ulcer, ulcerative colitis, functioning colostomy etc.
- Continued blood loss which cannot be corrected
- Impaired absorption which cannot be corrected.
- When rapid replenishment of stores is required – Ex – Pregnancy.
- Simultaneously along with erythropoietin
- Contraindication: History of allergy/ anaphylaxis
- Options of treatment include:
- Inj. Ferric carboxy maltose- 500mg in 100ml NS over 30min OD for 2-3 days (Based on degree of anemia and weight of patient)
- Iron sucrose (Polynuclear iron hydroxide in sucrose): 100-200mg/day, to a total cumulative dose of 1000mg
- Iron sorbitol - IM by Z technique
- Iron dextran: Ganzoni equation- Total requirement in mg= (Normal Hb - Patient’s Hb) X Weight in kg X 2.21 + 1000mg
- Sodium ferric gluconate
- Indications for parenteral therapy.
- Packed cell infusion : Indication
- Patients with cardiovascular instability
- Continued blood loss from whatever source
Monitoring After Treatment/ Follow-up:
- Upon treatment with iron (Oral/ Parenteral)
- Reticulocyte count increase by 3rd day and peaks by 10th day.
- Hb raises by 1g/dL in every week (0.15g/dL per day) and restored to normal by 6-10 weeks
- Oral therapy should be continued for 3-6 months after restoration of normal hematological values to correct deficits in iron stores.
- Encourage to eat diversified diet
Special situations:
- Iron deficiency in pregnancy:
- It is the most common cause of anemia in pregnancy. 75% of anemia during pregnancy are due to iron deficiency.
- S. Ferritin level of <30microg/l in pregnancy is indicative of iron deficiency
- Problems for mother
- Increased susceptibility to infections
- Poor work capacity
- Increased risk of post-partum hemorrhage
- Increased risk of post-partum depression
- Increased risk of mortality
- Effects on fetus
- Impaired psychomotor and mental development
- Low birth weight
- Preterm birth
- For routine prophylaxis-
- Oral iron is cheap, safe and effective way of replacement of iron.
- Recommended dose of elemental iron is 30-100mg daily
- T. Livogen-OD is sufficient (Ferrous fumarate 152mg= 47mg of elemental iron, with folic acid- 1.5mg).
- Recent studies have shown lower doses (40-80mg) given on alternate days are equally effective and have lower side effects due to less amount of unabsorbed iron in the gut. There is better absorption by alternate day dosage due to increased levels of hepcidin.
- Avoid prophylaxis only if hemoglobin is >13.2gm/dL with normal ferritin and in patients with known hemoglobinopathy with high ferritin levels.
- Iron replacement is absolutely necessary if
- Previous anemia
- Multiparity- P>2
- Twin or higher order multiple pregnancy
- Interpregnancy interval of <1 year
- Women who have poor dietary habits
- Those following vegetarian diet
- Repeat hemoglobin after 2-3 weeks to assess the response. Once hemoglobin is in normal range, replacement must continue for 3 months and until at least 6 weeks postpartum to replenish iron stores.
- If response to iron replacement is poor, in spite of good compliance, one must look for alternate causes of anemia such as folate deficiency and malabsorption.
- If not tolerating/ proven malabsorption/ rapid replenishment is required/ poor compliance to oral iron- Inj. Ferric carboxymaltose 500mg in 100ml NS over 15min OD for 2 days (Total dose is 20mg/Kg) can be given in second trimester. Avoid IV iron if there is history of serious reaction/ first trimester of pregnancy/ active bacteremia/ decompensated liver disease.
Figures:

Figure 8.1.1- Koilonychia
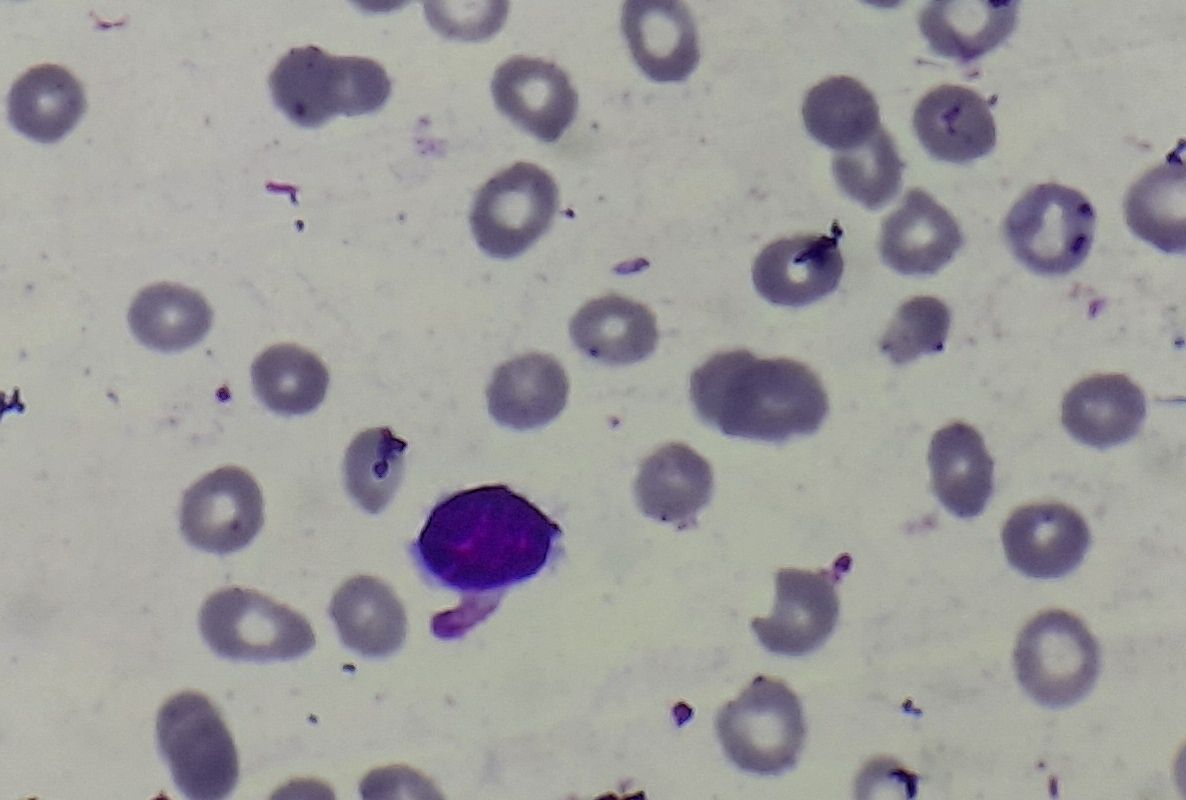
Figure 8.1.2- Iron deficiency anemia- Peripheral smear
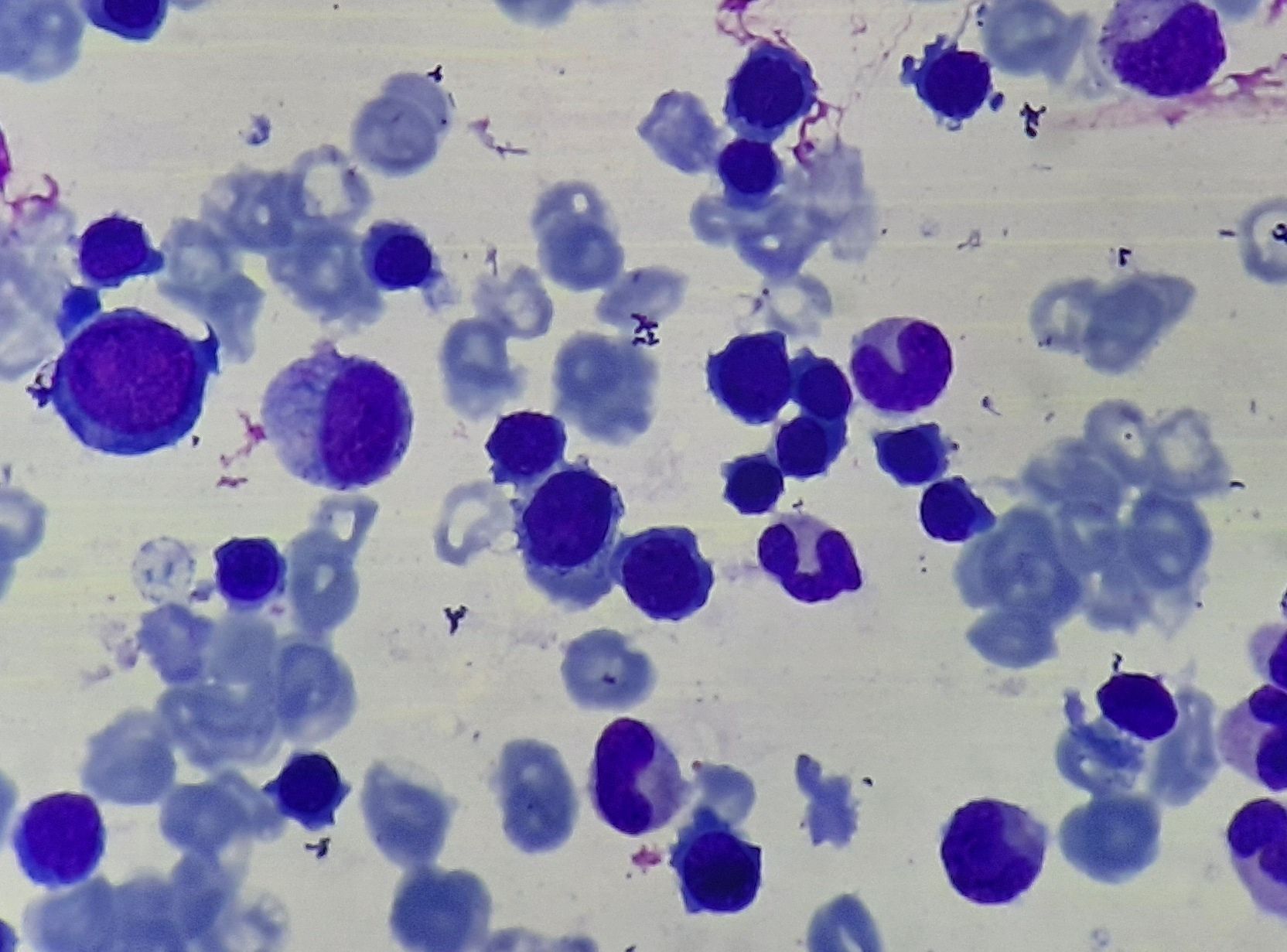
Figure 8.1.3- Iron deficiency anemia- Bone marrow aspiration
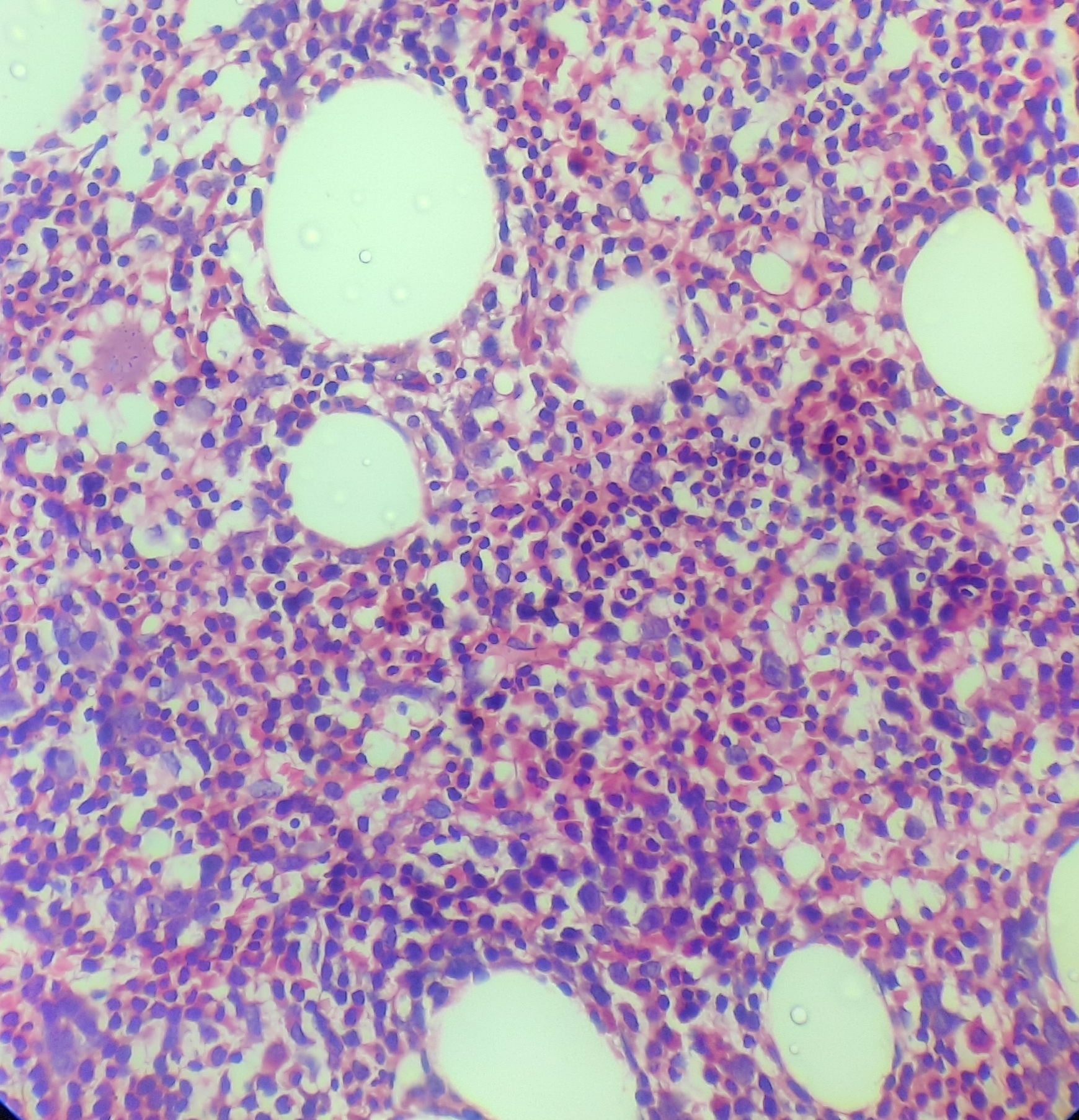
Figure 8.1.4- Iron deficiency anemia- Bone marrow biopsy
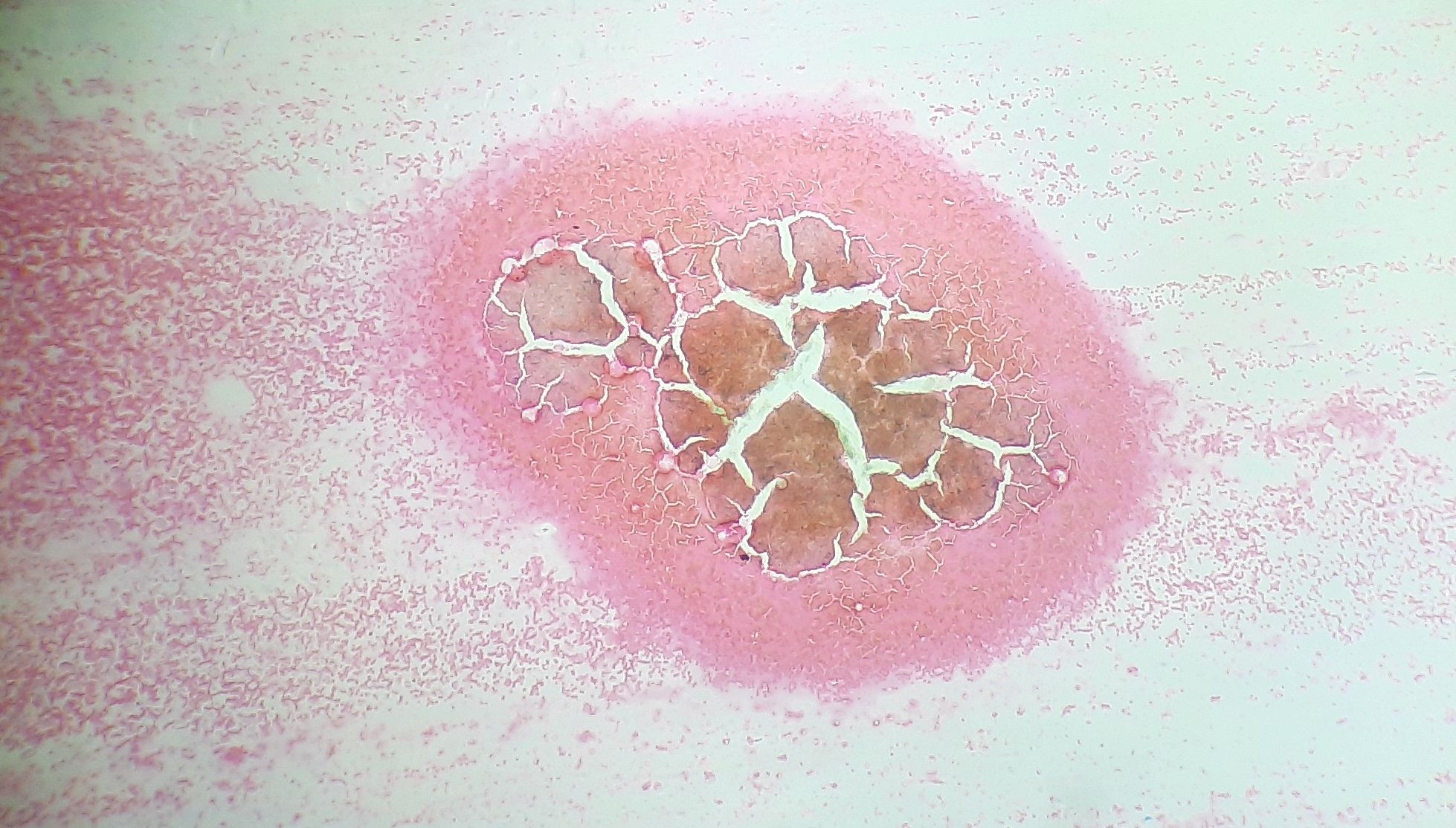
Figure 8.1.5- Iron deficiency anemia- Depleted iron stores in bone marrow aspirate
Recent advances:
Intravenous Iron versus oral iron for the treatment of restless legs syndrome in patients with iron deficiency anemia
This study compared the efficacy and speed of response between oral ferrous sulfate and intravenous ferumoxytol for treating Restless Legs Syndrome (RLS) in patients with iron deficiency anemia (IDA). A double-blind, double-dummy trial aimed to recruit 70 patients but faced challenges due to the COVID-19 pandemic, leading to missing final-week data for 30 patients and necessitating the recruitment of an additional 30 patients. At Week 6, primary outcomes measured were the Clinical Global Impression—Improvement score and changes in the International Restless Legs Syndrome Study Group rating scale score. Both IV and oral iron treatments showed significant improvement in RLS symptoms, with no significant difference between the two groups and no serious adverse events reported.
https://doi.org/10.1002/ajh.27290
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.