howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Lymphoplasmacytic Lymphoma/ WaldenstormMacroglobulinemia
Introduction:
- Lymphoplasmacytic lymphoma is a neoplasm of small B-lymphocytes, plasmacytoid lymphocytes and plasma cells, usually lacking CD5 and monoclonal serum M protein.
- Waldenstrom macroglobulinemia is LPL with bone marrow involvement and IgM monoclonal gammopathy of any concentration.
Epidemiology:
- Incidence- 3.4/1 million
- 3-7/1million women
- Accounts for 1.5% of nodal lymphomas
- Median age- 60yrs
- Slight male preponderance
- Familial predisposition is seen
Etiology:
- Infection – HCV
- Genetic susceptibility
- Occupational exposure
- Common in families with history of autoimmune disorders
Pathogenesis:
- MYD 88 mutation is seen in nearly 90% of LPL/WM
- It is a key component in Toll like receptor signalling machinery
- Mutation leads to gain of function
- Ibrutinib (BTK inhibitor) and Idalalisib (PI3K delta inhibitor) block MYD 88 mediated down-stream targets.
- Mutations can be detected by next generation sequencing/ PCR
Most common mutation is L265P
↓
Conformation change in beta loop of TIR domain
↓
Spontaneous homodimerization and recruitment of IRAK1 and IRAK4
↓
Uncontrolled formation of MYD 88/IRAK complex
↓
Recruitment of TRAF6 constitutive phosphorylation of PAK1
↓
Elevation of NF Kappa beta activity
↓
Sustained tumor cell survival and proliferation
- Mutation of CXCR4- Seen in 30-40% of patients. Confers resistance to Ibrutinib therapy.
- TP53 mutation- Seen in 11% of patients. Associated with poor prognosis.
Subtypes:
- IgM type
- Non- IgM type- 5% cases, IgG/ IgA/non-secretory, less frequent hyperviscosity/ neuropathy symptoms
Clinical Features:
- Lymphadenopathy: Develops slowly over years
- Hyperviscosity symptoms due to increased IgM which may form aggregates and may bind water through their carbohydrate component and induction of red cell aggregation/ rouleaux formation. Symptoms are seen when IgM is >50gm/L or Viscosity is >4 centipoise
- Headache
- Oronasal mucosal bleed
- Visual disturbances/ blurred vision
- Leg cramps
- Impaired mentation
- Dizziness
- Anemia- Due to
- Cold agglutinin hemolytic anemia due to anti PR2 cold agglutinin
- Hyperviscosity leads to reduced synthesis of EPO from kidney
- Infiltration in bone marrow
- Increased hepcidin levels
- Autoimmunity – Due to cryoglobulinemia
- Neuropathy – Due to reactivity of IgM paraprotein with myelin sheath antigens. Ex: Myeline associated glycoprotein
- Sensory symptoms: Paresthesia, aching discomfort, dysesthesia, lancinating pain
- Imbalance, gait ataxia, leg muscle atrophy
- Other neuropathies include:
- Amyloid peripheral neuropathy
- CANOMAD syndrome: chronic ataxic neuropathy with ophthalmoplegia, M-protein, cold agglutinins and disialosyl ganglioside antibodies
- Cryoglobulin associated neuropathy
- Small fiber neuropathy
- Diarrhoea, malabsorption, obstruction and GI bleed – Due to deposition of IgM/amyloid in GIT.
- Coagulopathies- Due to binding of IgM to clotting factors, platelets & fibrin.
- Skin lesions:
- Bullous lesions - Due to deposition of IgM on basement membrane
- Papular lesions on extensor surfaces of extremities- Due to deposition of IgM in dermis (Schnitzler syndrome)
- Hepatosplenomegaly
- Type II cryoglobulinemia
- Seen in 20% of patients
- Acrocyanosis and necrosis of regions exposed to cold (Raynaud's phenomenon)
- Vasculitis leading to purpura
- Arthralgia
- Fever
- Livido reticularis
- Lung-
- Masses, nodules, diffuse infiltrates, pleural effusion
- Presents with cough, dyspnea, chest pain
- Renal
- Renal/ perirenal masses
- Proteinuria- Due to deposition of IgM in subendothelial region of glomerulus
- Renal failure
- Joints
- Invasion of articular and periarticular structures
- Eyes
- Invasion of periorbital structures, lachrymal gland, retro-orbital lymphoid tissue leading to ocular nerve paralysis
- CNS
- Bing Neal syndrome- Due to direct invasion of CNS. There is confusion, memory loss, disorientation, and motor dysfunction
- Amyloidosis: Unexplained cardiac failure, intestinal dysmotility, purpura, macroglossia
Investigations:
- Hemogram
- Normocytic normochromic anemia
- False high MCV due to aggregation of RBCs
- False high haemoglobin due to interaction with paraproteins
- Mixture of small lymphocytes, plasma cells & plasmacytoid lymphocytes are seen, but count is much lesser than CLL
- Neutropenia and thrombocytopenia in late cases
- Bone marrow aspiration and biopsy
- Neoplastic cells- Small lymphocytes, plasmacytoid lymphocytes (Have abundant basophilic cytoplasm) and plasma cells, with / without PAS positive intranuclear inclusions (Dutcher bodies)
- Paratrabecular nodular lymphoid aggregates and / or a diffuse interstitial lymphoid infiltration.
- Increased number of reactive mast cells and hemosiderin laden histiocytes.
- Lymph Node biopsy
- Diffuse (without pseudofollicles) growth of tumor cells.
- Immunophenotyping
- Positive - surface Ig (IgM type) with light chain restriction, B cell associated antigens (CD19, CD20, CD22-weak CD79a, PAX5, FMC7), CD45, CD 38, CD25
- Negative –IgG, CD5, CD10, CD23 (CD5 is important & it helps in differentiating it from CLL), CD 103
- ESR- Increased
- Coagulation profile- Prolonged thrombin time
- Test for cold agglutinin/ cryoglobulin- May be positive
- Urine Bence Jones proteins- Frequently present
- Serum electrophoresis combined with Immunofixation (Serum and Urine) – Raised levels of IgM paraprotein (known as macroglobulin). Quantification must be done by densitometry/ nephelometry.
- S. Free light chain assay: Useful in conditions in which IgM component is difficult to measure (Ex: Cryoglobulins)
- Serum Viscosity
- Cytogenetics/FISH/ Molecular studies
- MYD88 p.L265P by targeted next generation sequencing- Positive in 93-97% cases
- IgH translocations are not seen- This helps to differentiate from multiple myeloma and follicular lymphoma
- Variable region may show somatic mutations
- Loss of all or part of chromosome- 17, 18, 19, 20, 21, 22, X and Y
- Gains of 3, 4 and 12
- t (9 : 14)- Rearrangement of PAX gene on chromosome 9 which encodes B-cell specific activator protein.
- del 6q, trisomy 3 and 18, trisomy 4
- Increased BCL2 expression
- MRI spine and CT abdomen and pelvis- To evaluate disease status
- Ophthalmoscopy- Distended, tortuous retinal veins, haemorrhages and papilledema
Criteria for Diagnosis
Lymphoplasmacytic lymphoma
Essential:
- Significant BM infiltration by clonal small lymphocytes with plasmacytoid and/or plasma cell differentiation
- Immunophenotype of LPL cells: IgM+, CD19+, CD20+, CD22+, CD25+, CD10-, CD23-, CD103-, CD138+/-
Desirable:
- Detection of MYD88 (NP_002459.2:p.L265P)
- Detection of CXCR4 somatic mutation.
- Serum electrophoresis and immunofixation showing presence of monoclonal IgM
Waldenstrom's macroglobulinemia:
- IgM monoclonal protein of any value in serum
- Bone marrow lymphocytes- ≥ 10% with plasmacytoid differentiation
- Diffuse, interstitial or nodular pattern of bone marrow infiltration
Prognosis:
- Poor prognostic factors
- Advanced stage
- Peripheral blood cytopenia especially anemia
- Poor PS
- High beta 2 microglobulins
- Increased transformed cells/ immunoblasts
- 6q deletion.
- Median survival – 5-10 years
- Transformation into diffuse large B-cell lymphoma may be seen
- No treatment can cure the disease
- International prognostic scoring system for WM- Each factor- 1 point
- Age- >65yrs
- Hemoglobin- <11.5gm/dL
- Platelet count <1lac/cmm
- Beta2microglobulin- >3mg/L
- IgM>7gm/dL
Score | 5 year survival |
0-1 | 87% |
Age >65 or 2 | 68% |
3-5 | 36% |
Indications for Treatment:
- Hyperviscosity related symptoms
- Neuropathy
- Organomegaly
- Amyloidosis
- Cold agglutinin disease
- Cryoglobulinemia
- Anemia/ Cytopenia secondary to marrow infiltration
- Bulky lymphadenopathy
- Evidence of end organ damage
Pretreatment Work-up:
- History
- Examination
- LN:
- Spleen:
- Fundoscopy:
- WHO P. S.
- BSA
- BMA and Bx
- IHC/Flow cytometry
- CT (CAP) or PET-CT (If agressive histology noted in histology)
- Hemoglobin
- TLC, DLC
- Platelet count
- LFT- Bili- T/D SGPT: SGOT: Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K: Ca:Mg: PO4:
- Uric acid:
- Quantitative Ig
- S.P.E.
- Immunofixation E
- SFLC Assay
- Beta 2 Microglobulin
- S. Viscosity
- Cryocrit (If cryoglobulinemia is suspected)
- Cold agglutinins
- DCT
- Anti MAG antibodies, Electromyelogram and Abdominal fat biopsy for Amyloid in pts with peripheral neuropathy
- Retinal examination- To note hyperviscosity (If IgM>3gm)
- CSF analysis (In patients with CNS manifestations)
- LDH
- HIV:
- HBsAg:
- HCV:
- UPT
- MYD88, L265P- PCR on BMA
- CXCR4 if Ibrutinib is considered
- Prognostic score
- ECHO(If anthracyclines planned)LVEF- %
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
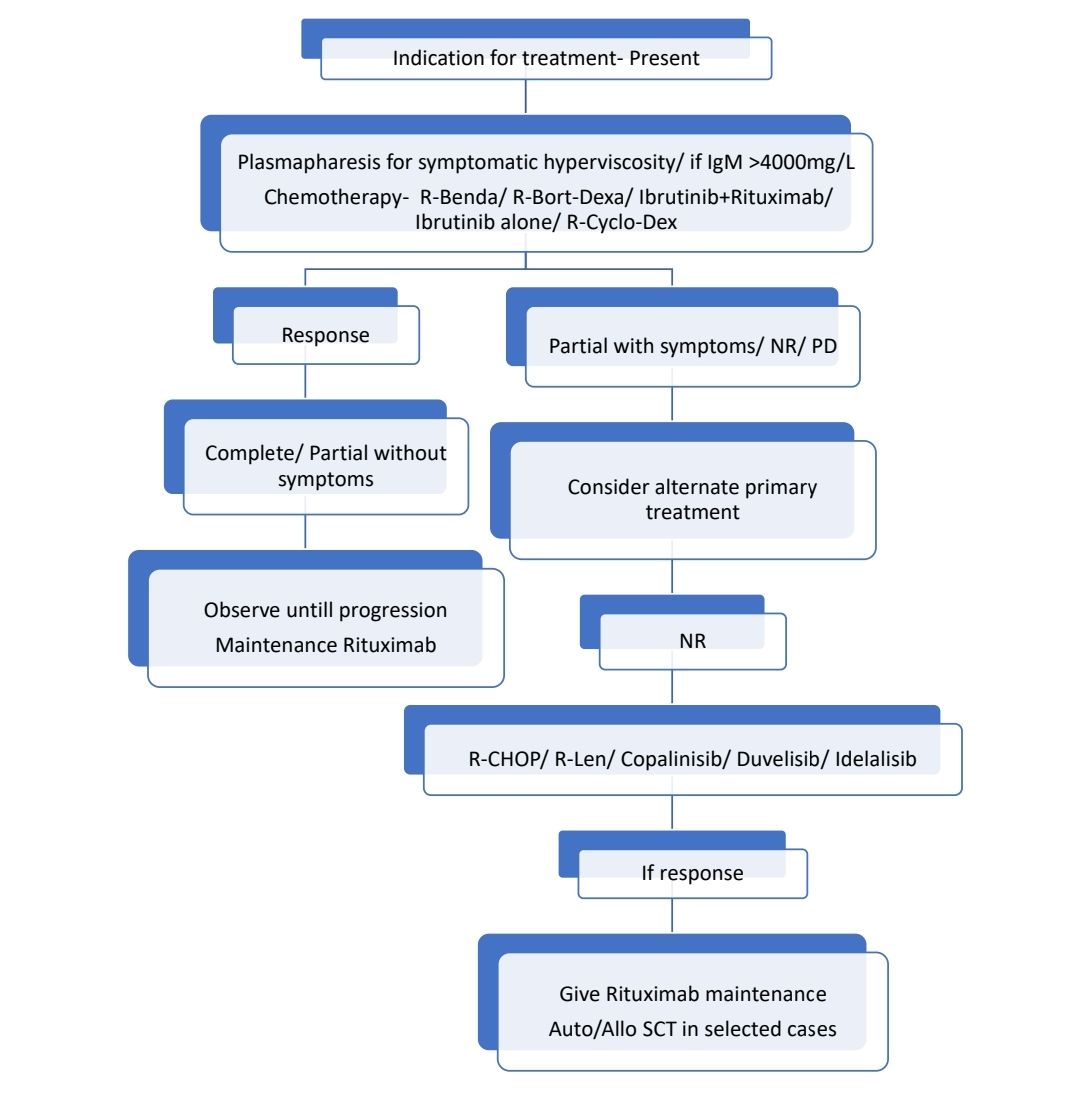
Treatment Plan:
- Intent of treatment must be palliation of symptoms, not necessarily normalizing IgM levels.
Response Criteria:
- Complete response:
- IgM in normal range and disappearance of monoclonal protein by immunofixation
- No histological evidence of BM involvement
- Resolution of lymphadenopathy and splenomegaly
- No symptoms attributable to WM.
- Partial response:
- >50% reduction in S. IgM levels
- Decrease in lymphadenopathy and splenomegaly
- No new symptoms or signs of active disease
About Each Modality of Treatment:
- Plasmapheresis:
- Should be performed if patient has symptomatic hyperviscosity
- If patient has IgM >4000mg/dL, plasmapheresis should be done prior to administering Rituximab/ Ofatumumab, as they cause IgM flare.
- Chemotherapy:
- Choice of frontline therapy depends n
- Presence of cytopenia
- Need for more rapid disease control
- Age and fitness for autologous transplantation (In candidates of autologous transplantation, avoid chlorambucil and nucleoside analogues)
- Avoid Bortezomib and vincristine in patients having significant peripheral neuropathy
- Subcutaneous route is preferred method of administration of Bortezomib
- Rituximab/Ofatumumab:
- They may be withheld in patients with elevated IgM levels for initial treatment cycles
- IgM flare occurs due to release of IL-6 by bystander immune cells. Return to baseline IgM is seen in 12 weeks.
- Ibrutinib
- Dose: 420mg- OD- Lifelong
- Risk of atrial fibrillation- 12-20%
- No response if patient has wild type MYD88
- Choice of frontline therapy depends n
- Rituximab maintenance:
- Given to only those patients who respond to rituximab containing regimens
- 375mg/m2 given, once in 3 months for 2 years
- Autologous stem cell transplant
- Done after HDT with Melphalan- 200mg/m2
- Useful in relapse/ refractory states
- May be used as first line therapy if there is associated amyloidosis
Other treatment options:
- Zanubrutinib
- Acalabrutinib
- Pirtobrutinib
- Venetoclax
Supportive Care:
- PCP prophylaxis is must for patients receiving bendamustine
- Herpes zoster prophylaxis has to be given to all patients receiving therapy.
- Yearly influenza and COVID-19 vaccinations must be given
- Pneumococcal vaccine
- Inappropriate PRBC transfusion can increase hyperviscosity and precipitate cardiac failure. PRBC transfusion can be done after plasma exchange.
Monitoring After Treatment/ Follow-up:
- IgM every 3 months for 3 years, then once in 6 months for 3 years, then once a year
- Progression based on IgM levels alone, without symptoms, is not an indication for retreatment
Treatment of relapse:
- Relapse within 12 months of completion of initial therapy: Choose alternate therapy
- Relapse after 12 months: Use previous treatment
Related conditions:
IgM monoclonal gammopathy of undetermined significance
- All the following criteria must be met
- the presence of an IgM paraprotein of less than 30 g/L
- Absence of a lymphoplasmacytic bone marrow infiltration
- Absence of signs or symptoms such as occur in WM itself
- No treatment is required
Histological transformation to DLBCL:
- Seen in 3-11% patients
- Associated with very high incidence of involvement of extranodal disease
- PET directed tissue biopsy is required for all patients
- Treatment is similar to denovo DLBCL
- Autologous SCt must be considered in patients achieving CR and are fit for high dose chemotherapy.
Figures:
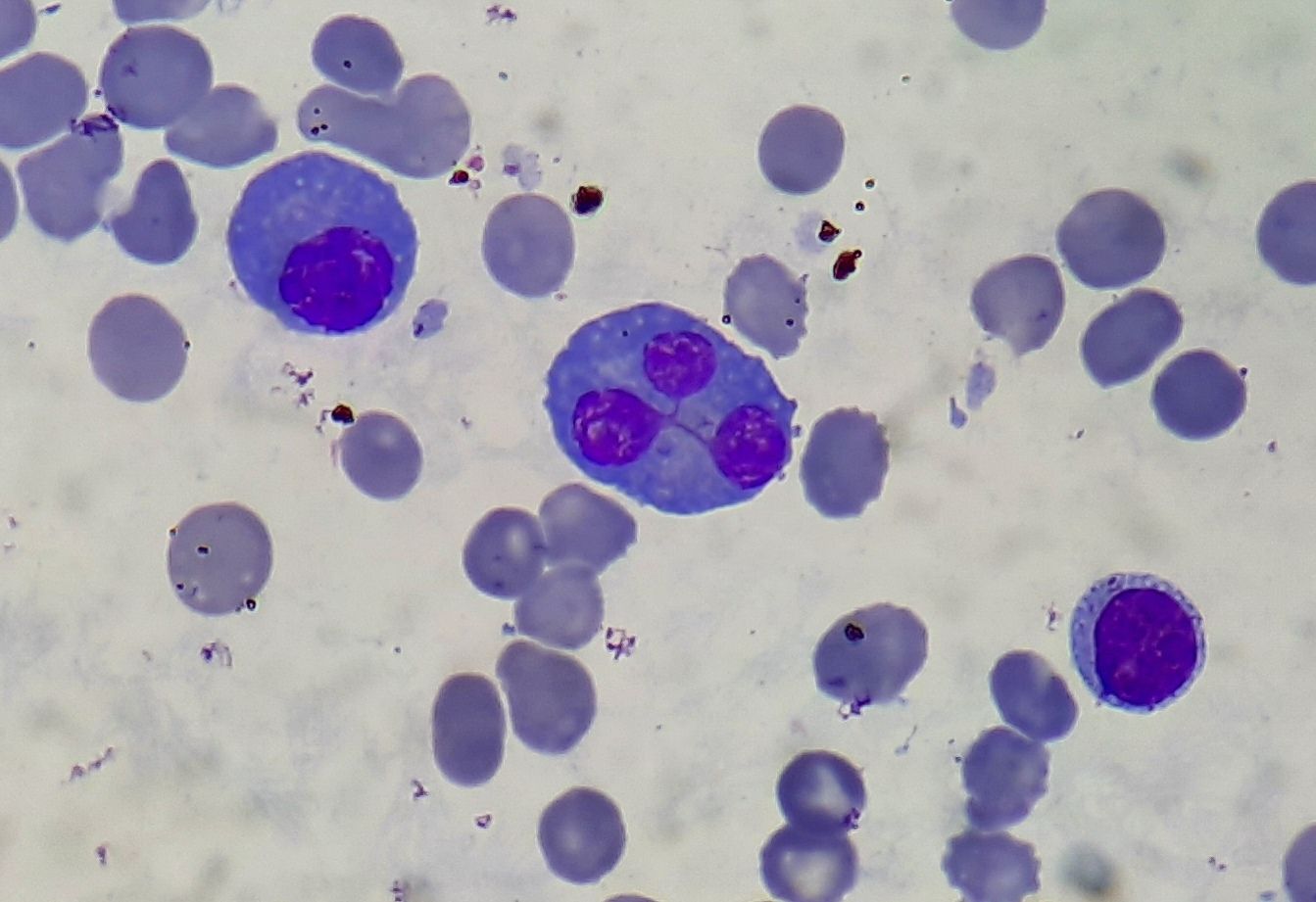
Figure 6.6.1- Lymphoplasmacytic lymphoma- Peripheral smear showing both lymphocytes and plasma cells
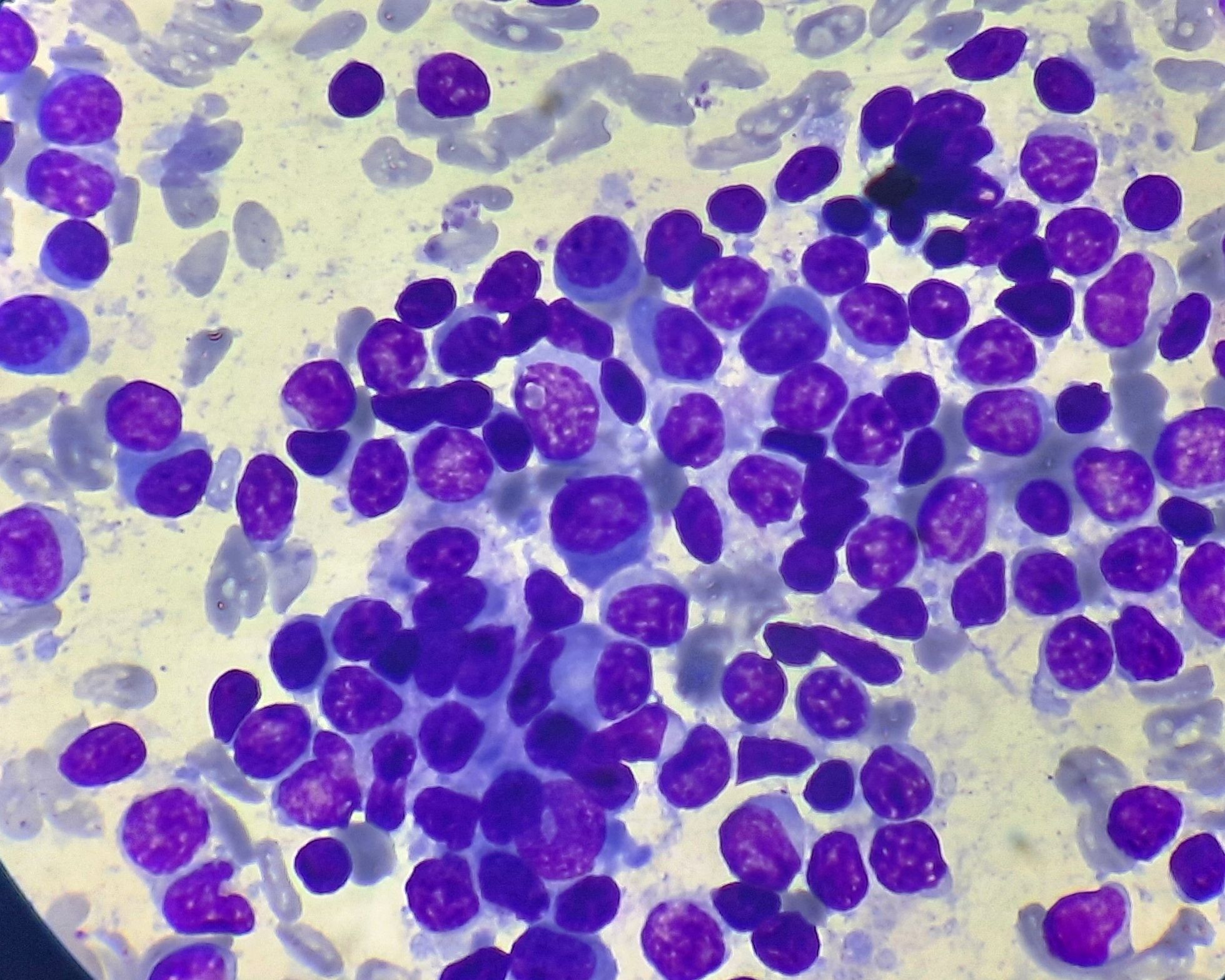
Figure 6.6.2- Lymphoplasmacytic lymphoma- Bone marrow aspiration
Recent advances:
Ibrutinib and venetoclax as primary therapy in symptomatic, treatment-naïve Waldenström macroglobulinemia
In this investigator-initiated trial, ibrutinib and venetoclax combination therapy was evaluated in symptomatic treatment-naïve patients with MYD88-mutated Waldenström macroglobulinemia (WM). Forty-five patients received ibrutinib followed by venetoclax, with the primary endpoint being the attainment of very good partial response (VGPR). The study demonstrated a high VGPR rate of 42%, with manageable adverse events including neutropenia, mucositis, and tumor lysis syndrome, but notable incidences of atrial fibrillation and ventricular arrhythmia. Progression-free survival (PFS) and overall survival (OS) rates at 24 months were favorable, although ventricular arrhythmia led to early termination of the study due to safety concerns.
https://doi.org/10.1182/blood.2023022420
Long-term results of Waldenström macroglobulinaemia treatment by bendamustine and rituximab
The bendamustine–rituximab (BR) regimen is a highly effective first-line therapy for Waldenström macroglobulinaemia (WM), with a previous analysis of 69 patients showing high response rates and favorable progression-free survival (PFS) and overall survival (OS). At a median follow-up of 76.1 months, 5-year PFS is 66.63% and OS is 80.01%. The incidence of secondary cancers is 17.66% at 66 months. Relapsed patients receiving ibrutinib as a second-line treatment benefited significantly, supporting the long-term efficacy of BR as a first-line treatment for WM.
https://doi.org/10.1111/bjh.19409
Simplified Risk Stratification Model for Patients With Waldenström Macroglobulinemia
This study reviewed 889 treatment-naïve Waldenström macroglobulinemia (WM) patients to identify prognostic factors for overall survival (OS). Significant predictors—age, serum lactate dehydrogenase (LDH), and serum albumin—were used to create a prognostic model, the Modified Staging System for WM (MSS-WM). The model stratified patients into four risk groups with distinct OS outcomes and was validated in a separate cohort. MSS-WM is a simple, clinically useful tool that reliably predicts prognosis in symptomatic WM patients.
https://doi.org/10.1200/JCO.23.020
Rituximab and pembrolizumab in relapsed/refractory Waldenström's Macroglobulinaemia
The PembroWM trial assessed pembrolizumab (PD1-Inhibitor) plus rituximab in relapsed/refractory Waldenström's Macroglobulinaemia (WM) post-chemoimmunotherapy and covalent BTK inhibitors. Among 17 patients, the 24-week overall response rate was 50%, with a median response duration of 11.6 months. Progression-free survival was 13.6 months, and treatment was well tolerated. This study highlights the safety and potential efficacy of PD-1 modulation in WM.
https://doi.org/10.1111/bjh.19706
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.