howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Mantle Cell Lymphoma
Introduction:
- It is a B cell tumor derived from the mantle zone of lymphoid follicle, composed of monomorphic, small to medium sized lymphoid cells with irregular nuclear contours.
- >95% cases are associated with t(11;14), which results in IGH::CCND1 fusion and overexpression of cell cycle protein cyclin D1.
- It has worst features of both high and low grade NHL. It has aggressive clinical course like high grade NHL. But is resistant to chemotherapy and relapses often like low grade NHL.
Epidemiology:
- Accounts for 3-10% of NHL
- Median age- 60-65 years
- Male predominance
Pathogenesis: Multistep mutagenesis
Germline ATM and CHK2 mutation in Naive B lymphocytes
↓
t(11:14) with IGH::CCND1 fusionresults in overexpression of cyclin D1
↓
Activation of cyclin D1 dependent kinase pathway
↓
Loss of cell cycle suppressive effect of RB1 and p27, resulting in cell growth and malignant transformation
↓
In Situ MCL
↓
ATM and CHK2 mutation leading to increased genomic instability
↓
Classical MCL
↓
p16/CDK4/Rb/ARF/MdM2/p53 mutations leading to increased proliferation
↓
Blastoid MCL
- Mutation of Cyclin D1 and CDK4 leads to defective phosphorylation of Rb gene
- ATM inactivation leads to genomic instability due to impaired DNA repair, which leads to additional mutations
Classification:
- Indolent/ Leukemic non-nodal MCL:
- Now considered as different disease entity
- Presents with splenomegaly and lymphocytosis with bone marrow involvement
- Generally no lymphadenopathy
- Usually asymptomatic
- Proliferative index- Low (Ki 67- <30%)
- Nuclear SOX-1- Negative
- Generally CD5 negative
- Generally lambda chain restriction
- Predominant usage of the IGHV1-8 gene, together with higher somatic hypermutation load
- Fewer genetic alterations
- Survival- 5-12 years
- Aggressive:
- Usually have lymphadenopathy
- Tumor cells show blastoid morphology
- Refractory to treatment
- Short survival
Clinical Features:
- Generalized lymphadenopathy
- Hepatosplenomegaly- Seen in 30-60% patients
- Bone marrow involvement- Anemia, thrombocytopenia- Seen in 50-80% patients
- B Symptoms- Seen in 50% patients
- Extranodal involvement- Seen in 20% patients
- Common- GIT (Polyposis coli leading to abdominal pain, diarrhea, intestinal obstruction, hematochezia), Liver
- Rare- Waldeyer ring, breast, lung, skin, soft tissue, salivary gland, orbit
- Leukemic involvement is common
- Spread to CNS (Rare)
Investigations:
- Lymph node biopsy
- Architectural destruction by monomorphic lymphoid proliferation with a vaguely nodular / diffuse / mantle zone growth pattern
- Tumor cells are small to medium sized. Nucleus has markedly irregular nuclear contours. Often cleaved, resembling centrocyte. They have moderately dispersed chromatin with conspicuous nucleoli.
- Hyalinised small vessels are seen
- Scattered single epithelioid histiocytes may be seen giving starry sky appearance
- Morphologic variants of mantle cell lymphoma
- Blastoid Variants
- Classic: Cells resemble lymphoblasts with dispersed chromatin and have high mitotic rate (> 10 / 10 HPF and usually at least 20 – 30 / 10 HPF).
- Pleomorphic: heterogeneous cell with large cleaved to oval nuclei and pale cytoplasm on Giemsa (or MGP) stain. Nucleoli may be prominent.
- Small round lymphocytes with more clumped chromatin, either admixed or predominant, mimicking small lymphocytic lymphoma.
- Prominent foci of cells with abundant pale cytoplasm resembling marginal zone or monocytoid B-cells and mimicking a marginal zone B-cell lymphoma; sometimes these paler foci may also resemble proliferation centers of a small lymphocytic lymphoma.
- Blastoid Variants
- Peripheral smear: Involvement is seen in 25% patients.
- Bone marrow aspiration and trephine biopsy: Involvement is seen in 50% patients
- Immunophenotyping (Flow cytometry/ IHC)
- Positive: Surface IgM/IgD, sometimes IgG and IgA with light chain restriction, CD5, CD19, CD20, CD79b, CD22, FMC7, CD43, Cyclin D1 (on IHC only), BCL1, BCL2, BOB-1, OCT-2, PAX-5
- Negative- CD10, CD23, BCL-6, CD25, CD30, CD103, CD138, EBV/EBER, EMA, HHV8
- SOX-11- Useful in differentiating indolent and aggressive varieties
- Variable- CD23, CD10
- Cyclin D1 negative MCL harbour CCND2 rearrangements and are positive for Cyclin D2 or Cyclin D3.
- Ki-67- <30% is associated with favorable prognosis
- Note: CLL is FMC negative, CD23 Positive, CD20 and Ig weak positive
- Molecular studies:
- Antigen receptor genes : Rearrangement of Ig heavy and light chains
- Variable region genes are unmutated (Consistent with pregerminal centre B-cell)
- Point mutation / deletion of ATM gene (Ataxia Telangiectasia mutated)
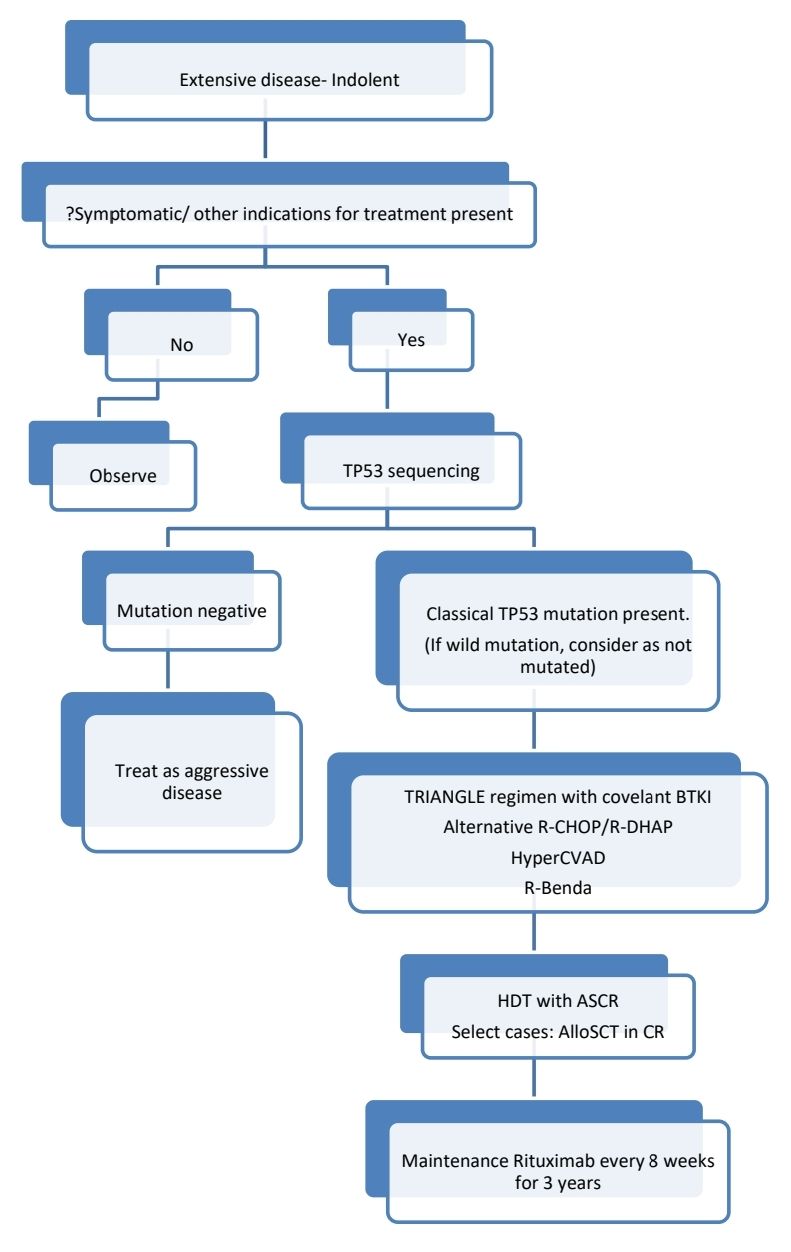
- Testing of TP53 by sequencing- To be done in patients with expected aggressive clinical course or particularly if upfront transplant is anticipated. It is associated with poor prognosis with conventional therapy, including transplant.
- SOX 11 or IGHV sequencing- May be useful in determination of clinically indolent MCL
- Some (blastoid type) have abnormalities in TP53, P16, P18
- Cytogenetics/ FISH
- t (11:14) - Between Ig heavy chain and Cyclin D1 genes (over expression of CYCLIN-D1 MRNA due to juxtaposition of BCL1 locus to IgH gene)
- Needed especially in cases of atypical morphology, aberrant immunophenotype, equivocal cyclin D1 positivity or unusual presentation.
- Other cytogenetic abnormalities:
- Gains of 3q26, 7p21, 8q24
- Losses of 1p13, 6q23, 9p21, 11q22, 13q11, 12p
- Degree of complexity is negatively associated with patient's survival.
Criteria for Diagnosis:
Cyclin D1-positive MCL
- Essential:
- Lymphoma cells of B-lineage (CD20-positive and usually CD5-positive)
- Morphology of classic variant (monomorphic and centrocyte-like) or less often variant morphology
- Cyclin D1-positive and/or detection of CCND1 rearrangement
- Desirable:
- SOX11 expression positive
Cyclin D1-negative MCL
- Essential:
- Lymphoma cells of B-lineage (CD20-positive and usually CD5-positive);
- Morphology of classic variant (monomorphic and centrocyte-like) or less often variant morphology
- Immunophenotype consistent with MCL including SOX11 expression
- Absence of cyclin D1 expression and CCND1 rearrangement
- Desirable:
- CCND2 rearrangement
Leukaemic non-nodal mantle cell lymphoma
- Essential:
- Typical asymptomatic clinical presentation (lymphocytosis; no or insignificant nodal involvement).
- Usually monomorphic small to medium-sized cells of B-lineage (CD20+)
- Cyclin D1 positive and/or detection of CCND1 rearrangement.
- Desirable:
- Absent or very weak expression of SOX11
Prognosis: Simplified MCL International Prognostic Index
Points | Age in yrs | ECOG PS | LDH (iU/L) | WBC Count (/cmm) |
0 | <50 | 0-1 | <0.67 | <6700 |
1 | 50-59 | - | 0.67-0.99 | 6700-9999 |
2 | 60-69 | 2-4 | 1-1.49 | 10,000- 14,999 |
3 | >69 | - | >1.5 | >15,000 |
Points | Risk category |
0-3 | Low |
4-5 | Intermediate |
6-11 | High |
- Median survival 3-5 years (Indolent type: 5-12 years)
- High incidence of relapse
- Poor prognostic markers
- Old age
- Poor PS
- CNS involvement at diagnosis
- High mitotic rate (>10-34 / HPF)
- High Ki-67- >30%
- Blastoid variant
- Peripheral blood involvement
- Trisomy 12
- Gain of 3q and deletion of 9q
- Complex karyotype
- P53 over expression by IHC
- TP53 mutation by NGS
- Increased beta 2 microglobulin, LDH
- Advanced stage (III or IV)
Pretreatment Work-up:
- History
- B-Symptoms
- Examination
- LN:
- Spleen:
- Liver:
- WHO P. S.
- BSA
- IHC/Flow cytometry
- Peripheral smear
- BMA and Bx- If PB is not involved
- CT (CAP+Neck)/ PET
- U/L GI Scopies (For confirmation of Stage 1 or 2)
- Stage
- Hemoglobin
- TLC, DLC
- Platelet count
- LFT: Bili- T/D SGPT: SGOT: Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid:
- LDH
- β2 Microglobulin
- HIV:
- HBsAg:
- HCV:
- UPT
- Cytogenetics
- FISH- t (11;14)
- TP53 sequencing
- CSF (For Blastoid variant/ CNS Symptoms)
- Prognostic score
- ECHO (If anthracyclines planned) LVEF- %
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
Treatment Plan:
- Limited disease (Stage I or Contiguous Stage II, Non-bulky)
- ISRT- Assess response
- CR- Observe
- <CR- Treat like extensive disease
- ISRT- Assess response
- Extensive disease (Stage II- Non contiguous/ Bulky, Stage III, Stage IV)
- Planning is based on whether disease indolent or aggressive
- Indolent disease is
- Leukemic- non-nodal CLL like presentation with splenomegaly
- Morphologically blastoid morphology is not seen (Blastoid tumor cells are seen in case of aggressive disease)
- GI/ Blood-BM involvement only
- Low tumor burden
- Ki67- <10%
- SOX-11- Positive
- IGHV- Mutated
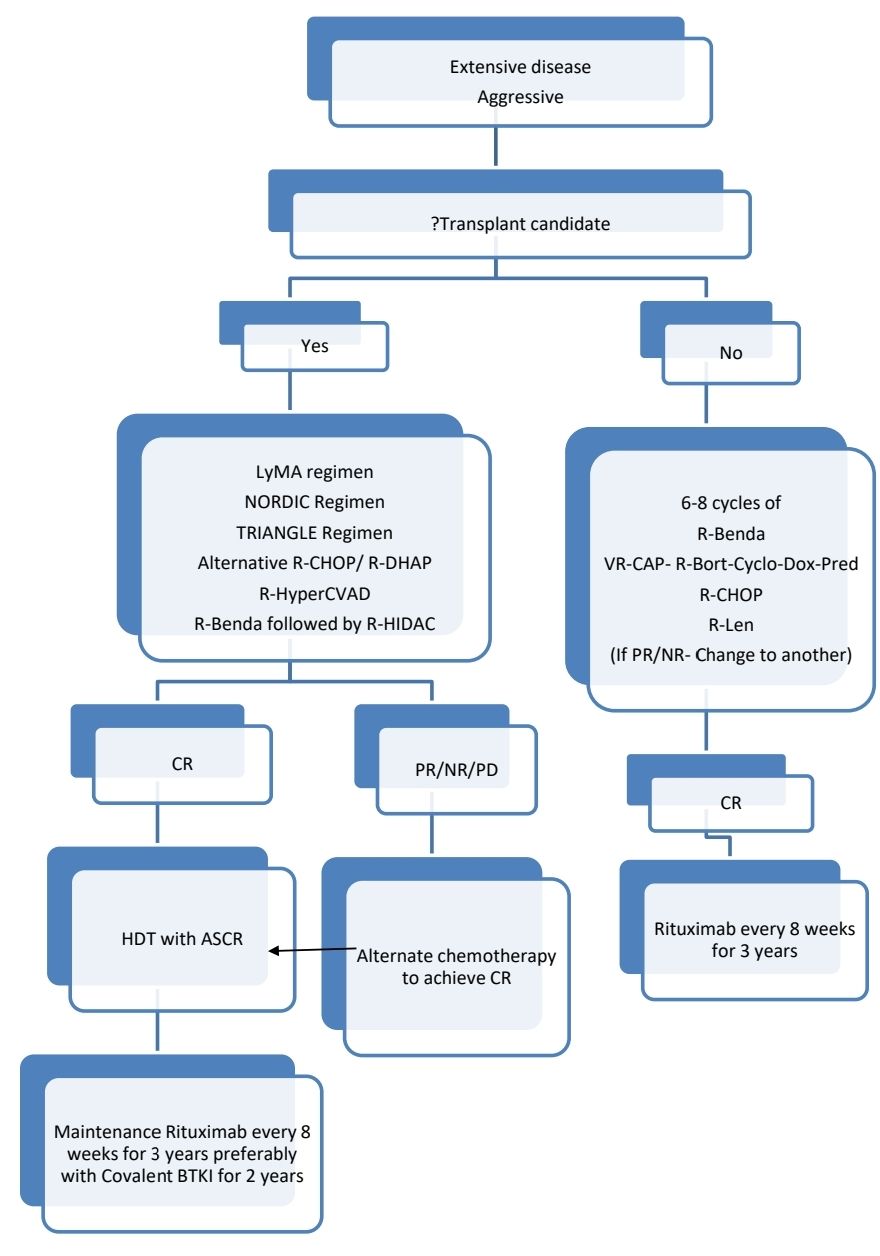
About each modality of treatment:
- LyMA regimen: RDHA (rituximab, dexamethasone, cytarabine) + platinum (carboplatin, cisplatin, or oxaliplatin) x 4 cycles followed by RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) for non-PET CR
- NORDIC regimen: Rituximab + cyclophosphamide, vincristine, doxorubicin, prednisone (maxi-CHOP) alternating with rituximab + high-dose cytarabine
- TRIANGLE regimen: Alternating RCHOP + covalent BTKig/RDHA (rituximab, dexamethasone, cytarabine) + platinum (carboplatin, cisplatin, or oxaliplatin)
- Covalent BTK inhibitors:
- Acalabrutinib
- Zanubrutinib
- Ibrutinib
- Non-covalent BTK inhibitors:
- Pirtobrutinib
- Anti CD19 CAR T-cell therapy
- Brexucabtagene autoleucel
Relapsed/ Refractory disease:
- Covalent BTK Inhibitors if not used so far and continue until progression
- Anti CD19 CAR T-cell therapy
- Non-Covalant BTKI
- Alternative Second line therapy followed by HDT/ASCR if not done previously
- Alternative Second line therapy followed by allogeneic SCT +/- ISRT
- Palliative ISRT/ Best supportive care
Monitoring After Treatment/ Follow-up:
- History, examination and labs, every 3-6 months for 5 years and then yearly or as clinically indicated.
- CT (C/A/P)- Every 6 monthly for 2 years and then annually.
Related Disorders:
- In situ Mantle cell lymphoma
- Presence of MCL like B cells (Cyclin D1 Positive) in the mantle zones of morphologically reactive lymph nodes
- No spread of Cyclin D1positive cells to interfollicular area
- Potential for malignancy in these patients is not known.
- Cyclin D1 negative MCL
- Lacks t(11:14)
- Morphology, immunophenotyping, clinical features, clinical course are similar to classical MCL
- Overexpression of Cyclin D2 or Cyclin D3 is noted with variant translocations
- Nuclear SOX11- Positive
- Treatment is same as classical MCL
Recent Advances:
Ibrutinib plus Bendamustine and Rituximab in Untreated Mantle-Cell Lymphoma
In a recent study elderly patients were given ibrutinib (560 mg, administered orally once daily until disease progression or unacceptable toxic effects) or placebo, plus six cycles of bendamustineand rituximab. Patients with an objective response received rituximab maintenance therapy, administered every 8 weeks for up to 12 additional doses. Among 523 patients, 261 were randomly assigned to receive ibrutinib and 262 to receive placebo. The median progression-free survival was 80.6 months in the ibrutinib group and 52.9 months in the placebo group. Overall survival was similar in the two groups.
https://doi.org/10.1056/NEJMoa2201817
Ibrutinib improves survival compared with chemotherapy in mantle cell lymphoma with central nervous system relapse
Central nervous system (CNS) relapse of mantle cell lymphoma (MCL) is a rare phenomenon with dismal prognosis, where no standard therapy exists. Ibrutinib is effective in relapsed/refractory MCL and penetrates the blood–brain barrier. Study concluded that Ibrutinib is associated with superior survival compared with BBB-penetrating chemotherapy in patients with CNS relapse of MCL and should be considered as a therapeutic option.
https://doi.org/10.1182/blood.2022015560
Pirtobrutinib in Covalent Bruton Tyrosine Kinase Inhibitor Pretreated Mantle-Cell Lymphoma
This study evaluated the safety and efficacy of pirtobrutinib, a reversible Bruton tyrosine kinase inhibitor (BTKi), in patients with mantle-cell lymphoma (MCL) who had been previously treated with covalent BTK inhibitors (cBTKi). In a phase I/II trial, 90 patients were included in the primary efficacy cohort. The overall response rate (ORR) was 57.8%, including 20.0% complete responses. The median duration of response was 21.6 months, with favorable rates at 6 and 12 months. Common adverse events included fatigue, diarrhea, and dyspnea, with few grade ≥3 events. Pirtobrutinib demonstrated durable efficacy and tolerability in cBTKi-pretreated MCL patients.
https://doi.org/10.1200/JCO.23.00562
Bendamustine–rituximab for older, untreated mantle cell lymphoma patients
In older patients (aged 60 and above) with mantle cell lymphoma (MCL) uniformly treated with bendamustine and rituximab, a comprehensive analysis was conducted to identify prognostic factors. The study revealed that blastoid cytology had an hazard ratio of 4.21 compared to classic cytology. Patients classified as high risk by both s-MIPI and MCL35 had the poorest prognosis, while those with high risk by either had a moderate but clinically relevant prognosis. The study suggests that a combination of s-MIPI, MCL35, and stringent cytological evaluation may effectively stratify risk in older MCL patients for future clinical trials.
https://doi.org/10.1111/bjh.19153
Real-world effectiveness and safety of ibrutinib in relapsed/refractory mantle cell lymphoma
This post-marketing surveillance study aimed to evaluate the efficacy and safety of ibrutinib in Japanese patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL). In the effectiveness analysis set (n = 202), the overall response rate was 59.9%, 52-week progression-free survival was 47.5%, and overall survival was 69.3%. Safety analysis in 248 patients (median age 74.0 years) showed an overall incidence of adverse events at 74.6%, with a similar distribution between patients with 1 versus more than 1 prior line of therapy. The most common adverse event was decreased platelet count (all grades; 10.4%). Atrial fibrillation occurred in 2.0% of patients.
https://doi.org/10.1007/s12185-023-03687-8
Predictive value of minimal residual disease for efficacy of Rituximab maintenance in mantle cell lymphoma
The study aimed to assess the impact of minimal residual disease (MRD) status on the efficacy of rituximab (R)-maintenance in older patients with mantle cell lymphoma (MCL) treated with immunochemotherapy. MRD monitoring was performed using quantitative polymerase chain reaction (qPCR) following EuroMRD guidelines. Results showed that R-maintenance was highly effective in patients who were MRD-negative after induction, leading to significantly improved progression-free survival (PFS) and overall survival (OS). However, MRD-positive patients had less benefit from R-maintenance, indicating the need for more effective consolidation strategies in this group to improve long-term outcomes.
https://doi.org/10.1200/JCO.23.0089
Lisocabtagene Maraleucel in Relapsed/Refractory Mantle Cell Lymphoma
The study presents primary analysis results from the TRANSCEND NHL 001 trial's mantle cell lymphoma (MCL) cohort, focusing on lisocabtagenemaraleucel (CD19 directed CAR T Cell) treatment in relapsed/refractory MCL patients. Of the 104 enrolled patients, 83 were included in the efficacy analysis. Lisocabtagenemaraleucel exhibited an objective response rate (ORR) of 83.1% and a complete response (CR) rate of 72.3%, with median duration of response and progression-free survival at 15.7 and 15.3 months, respectively. Adverse events (AEs) were mostly manageable, with neutropenia, anemia, and thrombocytopenia being the most common grade ≥3 treatment-emergent AEs. Notably, the incidence of severe cytokine release syndrome (CRS) and neurologic events (NEs) was low, indicating liso-cel's efficacy and tolerability in heavily pretreated R/R MCL patients.
https://doi.org/10.1200/JCO.23.022
Bortezomib, rituximab, high-dose cytarabine and dexamethasone in relapsed or refractory mantle cell lymphoma
In a phase-III trial for relapsed or refractory mantle cell lymphoma (MCL) patients ineligible for or relapsed after autologous stem cell transplant (ASCT), rituximab, high-dose cytarabine, and dexamethasone with bortezomib (R-HAD + B) demonstrated superior efficacy over R-HAD alone. Median time to treatment failure (TTF) was 12 vs. 2.6 months (p=0.045), with higher overall and complete response rates in the R-HAD + B group. Subgroup analysis revealed a significant treatment effect in patients >65 years and those without previous ASCT. Toxicity primarily comprised hematological adverse events, with more leukocytopenia and lymphocytopenia in the R-HAD + B group, indicating bortezomib's potential as a therapeutic option in r/r MCL.
https://doi.org/10.1038/s41375-024-02254-2
Rituximab, gemcitabine and oxaliplatin in relapsed or refractory indolent and mantle cell lymphoma
This phase I/II trial aimed to determine the maximum tolerated dose (MTD) of oxaliplatin in combination with rituximab and gemcitabine (R-GemOx) and evaluate its efficacy in relapsed or refractory (r/r) indolent lymphoma and mantle cell lymphoma (MCL). The MTD was established at 70 mg/m² of oxaliplatin after dose-limiting toxicities occurred at 80 mg/m². Among 46 evaluable patients, the overall response rate (ORR) was 72%, missing the primary study aim. After a median follow-up of 7.9 years, the median progression-free survival (PFS) was 1.0 year, and overall survival (OS) was 2.1 years. While R-GemOx showed decent efficacy, novel therapies have largely replaced chemotherapy in this setting, though R-GemOx may still be viable for late relapses or as bridging to CAR-T-cell therapy in MCL.
https://doi.org/10.1007/s00277-024-05689-w
Outcomes of venetoclax-ibrutinib therapy in mantle cell lymphoma
In this trial, 24 mantle cell lymphoma (MCL) patients (23 relapsed/refractory) received ibrutinib and venetoclax daily. After over 7 years of follow-up, the 7-year progression-free survival (PFS) was 30% and overall survival (OS) was 43%. Eight patients with MRD-negative complete remission (CR) underwent elective treatment interruption (ETI), with 4 experiencing disease recurrence. Two of 3 reattained CR on retreatment. Serious adverse events were rare beyond 56 weeks. The combination showed long-term durable responses with a favorable safety profile, supporting treatment interruption feasibility while maintaining disease control.
https://doi.org/10.1182/blood.2023023388
Carfilzomib, lenalidomide, dexamethasone (KRD) in BTKi relapsed or refractory mantle cell lymphoma
This phase II trial evaluated the carfilzomib-lenalidomide-dexamethasone (KRD) combination in relapsed/refractory mantle cell lymphoma (MCL) patients resistant or intolerant to Bruton's tyrosine kinase inhibitors (BTKi). The 12-month overall survival was only 13%, with an overall response rate of 19%. Grade 3–4 adverse events occurred in 35% of patients, leading to early trial termination.
https://doi.org/10.1111/bjh.19617
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.