howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Mastocytosis
Updated on 27.06.2025
Introduction:
- It is clonal proliferation of mast cells and their subsequent accumulation in one or more organ systems.
Epidemiology:
- Cutaneous usually affects children < 6 month. Regresses spontaneously at puberty.
- About 80% have only cutaneous involvement
- 20% have bone marrow involvement, when it is called as systemic mastocytosis
- Some of them are associated with other well defined myeloid malignancy such as MDS.
Etiopathogenesis:
- Often associated with activating KIT mutations (Codon 816 mutation in tyrosine kinase domain) which leads to ligand independent activation of tyrosine kinase. Hence Imatinib fails to act in this condition.
- Other somatic mutations include: TET2, DNMT3A, ASXL1, SF3B1, and CBL mutations.
- Upon mast cell degranulation, there is release of histamine, tryptase, IL-3, IL-16 and TNF. This leads to various systemic manifestations.
Clinical Features:
Cutaneous mastocytosis:
3 presentations:
- Maculopapular cutaneous mastocytosis: Exaggerated urticaria may lead to blistering lesions. Darier’s sign – Stroke over skin causes urticaria. Hyperpigmentation (Together it is called urticaria pigmentosa). Lesions are usually seen on trunk and proximal extremities. There are 2 subtypes:
- Polymorphic MPCM:
- Brown, yellow or pale red papules, plaques and/or nodules.
- Heterogeneity in number, shape, size, margination, and degree of confluence of the lesions.
- Monomorphic MPCM: Small, round, and pigmented macules and papules, ranging from few scattered lesions to generalized skin involvement with confluent lesions.
- Polymorphic MPCM:
- Diffuse cutaneous mastocytosis: Generalized erythroderma, thickened skin, exaggerated folds and episodic blistering often accompanied by severe systemic symptoms (Peauchagrine, grain lather skin)
- Mastocytoma: Usually solitary lesions, but up to 3 lesions are accepted. Yellow to brown papules, nodules, or plaques, often with leathery texture.
Systemic mastocytosis: Clonal proliferation of mast cells involving at least one extracutaneous organ system, with or without evidence of skin lesions.
Systemic manifestations: (Due to tissue release of histamine, eicosanoids, proteases and heparin)
- Constitutional symptoms- Fatigue, weight loss, fever, sweats, diaphoresis
- Skin Manifestations- Pruritus, urticaria- hives, angioedema, dermographism
- Mediator related events- Abdominal pain, GI distress (peptic ulcer disease, nausea, vomiting, diarrhoea), flushing of face, chest and neck, nasal itching and congestion, throat itching and swelling, headache, cognitive dysfunction, anxiety, syncope, hypertension/ hypotension, tachycardia, wheezing and shortness of breath
- Bone related (osteoporosis)- Bone pain, Fractures, Arthralgias
- Tissue infiltration- Splenomegaly, lymphadenopathy, hepatomegaly
- Hematological abnormalities- Anaemia, Leukocytosis, Eosinophilia (a common finding), Neutropenia, thrombocytopenia and bone marrow failure
Potential triggers for mast cell activation:
- Heat, cold, sudden temperature changes
- Stress: Physical, emotional
- Exercise
- Drugs: Opioids, antibiotics, local/systemic anaesthetics, contrast dyes
- Food including alcohol
- Natural odors, scent or perfumes
- Insect stings
- Venoms- Hymenoptera insects (comprising the sawflies, wasps, bees, and ants) spider, jelly fish, snakes
- Infections- Viral, bacterial, fungal
- Mechanical irritation, friction or vibration
- Sunlight
- Lack of sleep
- Surgery/ any procedures
- Vaccinations
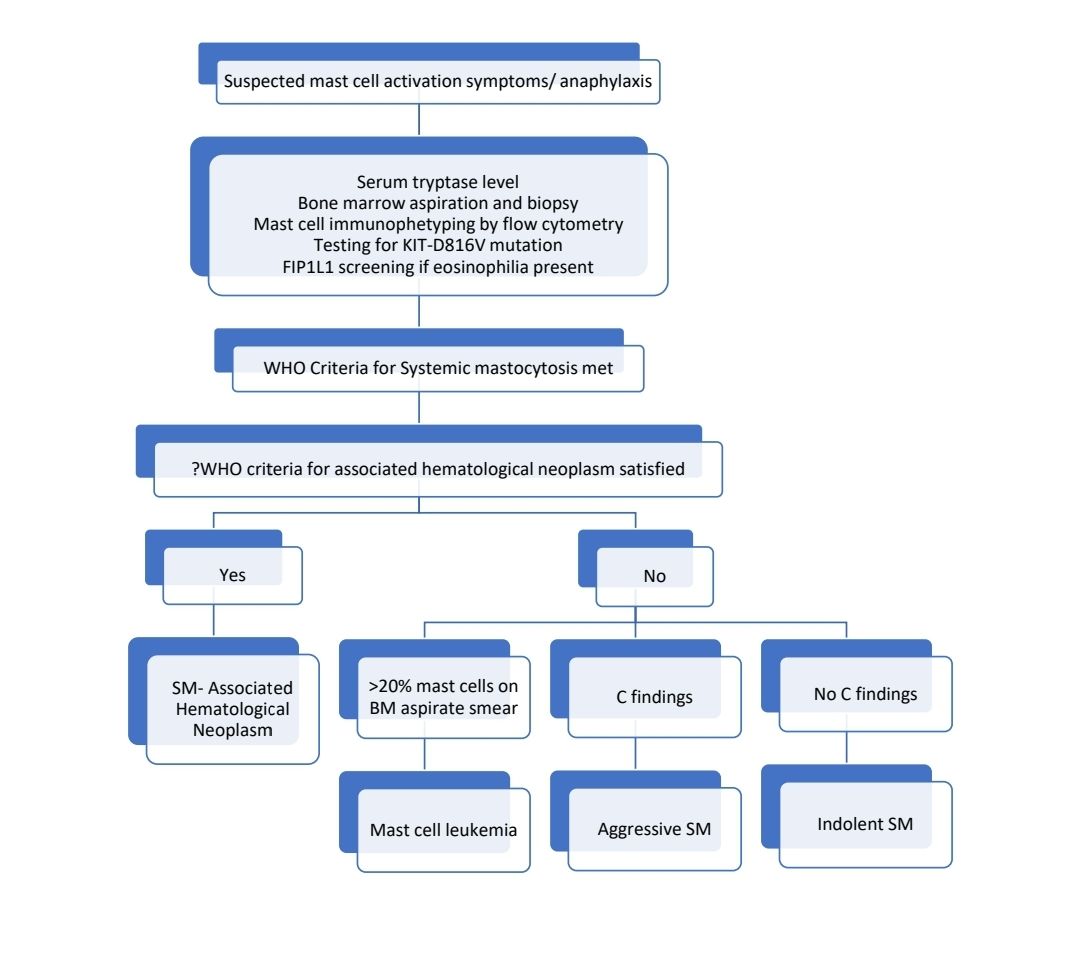
Investigations:
- Demonstration of multifocal clusters of mast cells in adequate biopsy
- Staining with Giemsa, toluidine blue or mast cell tryptase is strongly recommended
- Mast Cells in H&E appear as oval / polygonal shaped cells with round to oval nucleus and moderate amount of cytoplasm with eosinophilic granules.
- Bone marrow
- In aspirate samples mast cells appear as spindle shaped cells with abundant clear cytoplasm or histiocyte like polygonal cell with granular cytoplasm.
- They are found in peritrabecular and perivascular locations
- Focal lesions are comprised of varying proportions of mast cells, lymphocytes, eosinophils and fibroblasts
- Adjacent bone show significant fibrosis and thickening
- Sometimes associated hematopoietic malignancy may be noted (AML, MPN, MDS)
- When mast cells account for 20% or more of the cells in BM aspirate, then diagnosis of mast cell leukaemia may be suspected
- Lymph node biopsy
- Mast cell infiltration may be seen in any compartment
- Usually focal infiltrate involving paracortical areas is seen
- Associated with this there can be hyperplasia of germinal centers, small blood vessel hyperplasia, eosinophilia, plasmacytosis and collagen fibrosis
- Immunohistochemistry
- Usually positive- Mast cell tryptase, Naphthol ASD chloroacetate esterase, CD117, CD45, 33, 68, 117, CD9, CD43
- In contrast to normal mast cells CD2 and CD25 are expressed in neoplastic cells
- Usually negative- CD14, 15, 16, T-cell related antigens, B Cell related antigens
- If KIT is wild type and mast cell IHC is normal: Consider other causes for mast cell activation such as Allergies, Drugs, Infections, Idiopathic anaphylaxis and Hereditary alpha tryptasemia
- Hemogram
- Anemia
- Leucocytosis / Leukopenia
- Thrombocytopenia / thrombocytosis
- Eosinophilia
- Serum total tryptase- More than 20ng/ml in systemic mastocytosis
- Test for histamine metabolites in 24 hours urine specimen
- Plasma levels of soluble CD25 & CD117 (kit)
- USG and CT – To Look for hepatosplenomegaly and lymphadenopathy
- Bone densitometry – To look for presence of osteoporosis
- Molecular studies (PCR):Somatic point mutations of KIT (Encodes tyrosine kinase receptor for stem cell factor – SCF, mast cell GF) substitution of Val for Asp at codon 816.
Criteria for Diagnosis:
Cutaneous mastocytosis:
- Essential: Typical skin lesions demonstrating the typical clinical findings of urticaria pigmentosa, diffuse cutaneous mastocytosis or solitary mastocytoma and typical infiltrates of mast cell (CD117 positive) in a multi focal or diffuse pattern in an adequate skin biopsy. Should not meet criteria of systemic mastocytosis.
- Desirable: Aberrant immunophenotype (CD2 + CD25 + and / or CD30+); detection of KIT mutations in lesional skin.
Systemic Mastocytosis: One major and one minor criterion should be present, or three minor criteria are fulfilled.
Major Criteria
- Multifocal, dense infiltrates of mast cells (15 or more mast cells in aggregates) detected in sections of bone marrow and/or other extracutaneous organs, and confirmed by tryptase immunohistochemistry or other special stains.
Minor criteria
- In biopsy sections of bone marrow or other extracutaneous organs, more than 25% of the mast cells in the infiltrate are spindle – shaped or have atypical morphology, or of all mast cells in bone marrow aspirate smears, more than 25% are immature or atypical mast cells.
- Detection of KIT point mutation at codon 816 in bone marrow, blood or other extracutaneous organ
- Mast cells in bone marrow, blood or other extracutaneous organs that co-express CD117 with CD2 and / or CD25
- Serum total tryptase persistently > 20 ng/ml
Systemic mastocytosis is further classified into indolent, smoldering and aggressive types. For classifying into these categories one must know “B” and “C” findings.
“B” findings
- Bone marrow biopsy showing >30% infiltration by mast cells (focal, dense aggregates) and / or serum total tryptase level > 200 ng/ml.
- Signs of dysplasia or myeloproliferation, in non-mast cell lineage, but insufficient criteria for definitive diagnosis of a hematopoietic neoplasm by WHO, with normal or only slightly abnormal blood counts.
- Hepatomegaly without impairment of liver function, and / or palpable splenomegaly without hypersplenism, and / or palpable or visceral / lymphadenopathy.
“C” findings
- Bone marrow dysfunction manifested by one or more cytopenia (ANC < 1.0 x 109/dl, or platelets <100 x 109/L) but no frank non-mast cell hematopoietic malignancy.
- Palpable hepatomegaly with impairment of liver function, ascites, and / or portal hypertension
- Skeletal involvement with large sized osteolytic and / or pathological fractures
- Palpable splenomegaly with hypersplenism
- Malabsorption with weight loss due to GI mast cell infiltrates
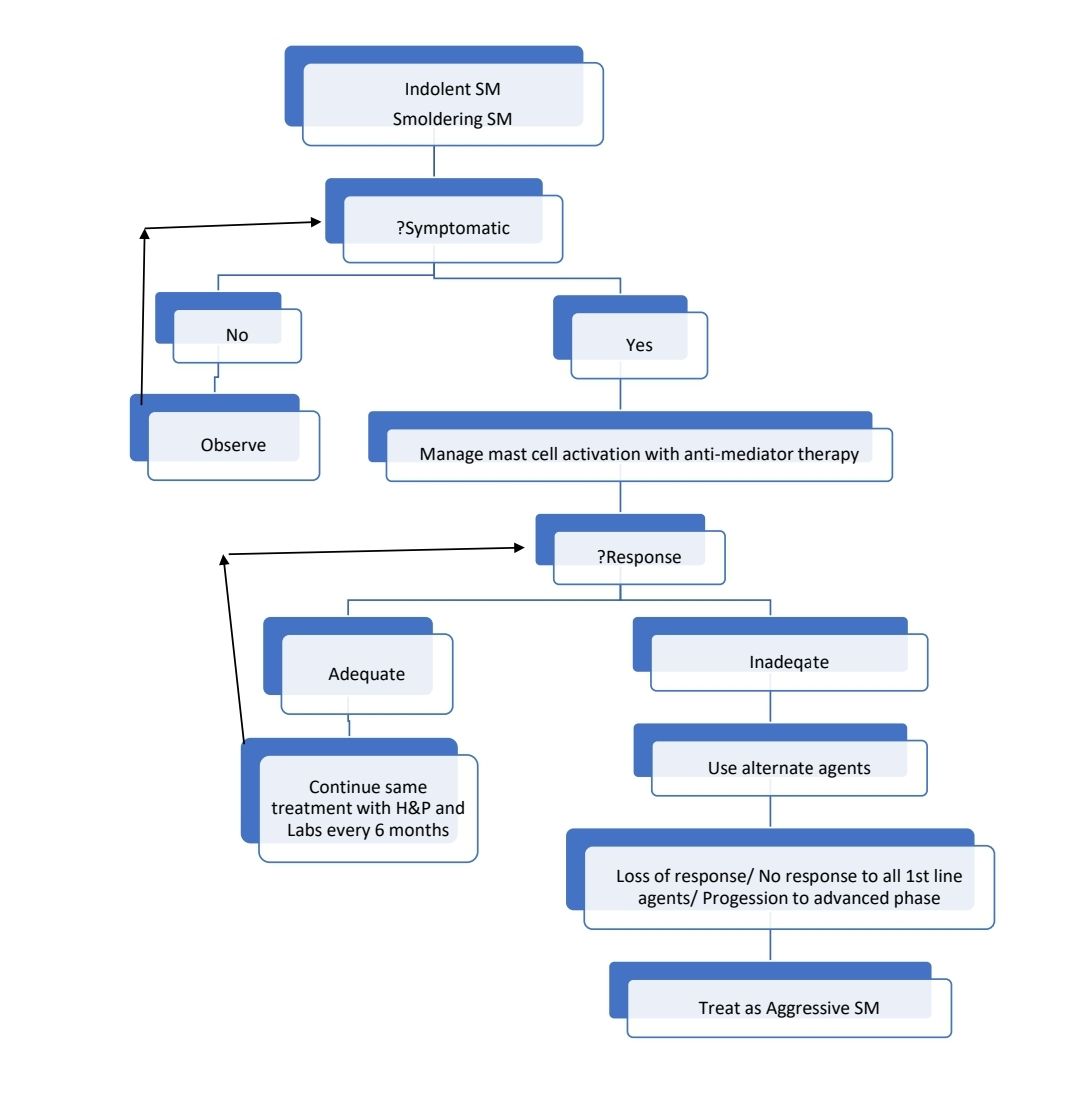
Indolent Systemic Mastocytosis
- No “B” or “C” findings
- Bone marrow mastocytosis - as above with bone marrow involvement, but no skin lesions
Smoldering systemic mastocytosis
- 2 or more “B” findings but no “C” finding
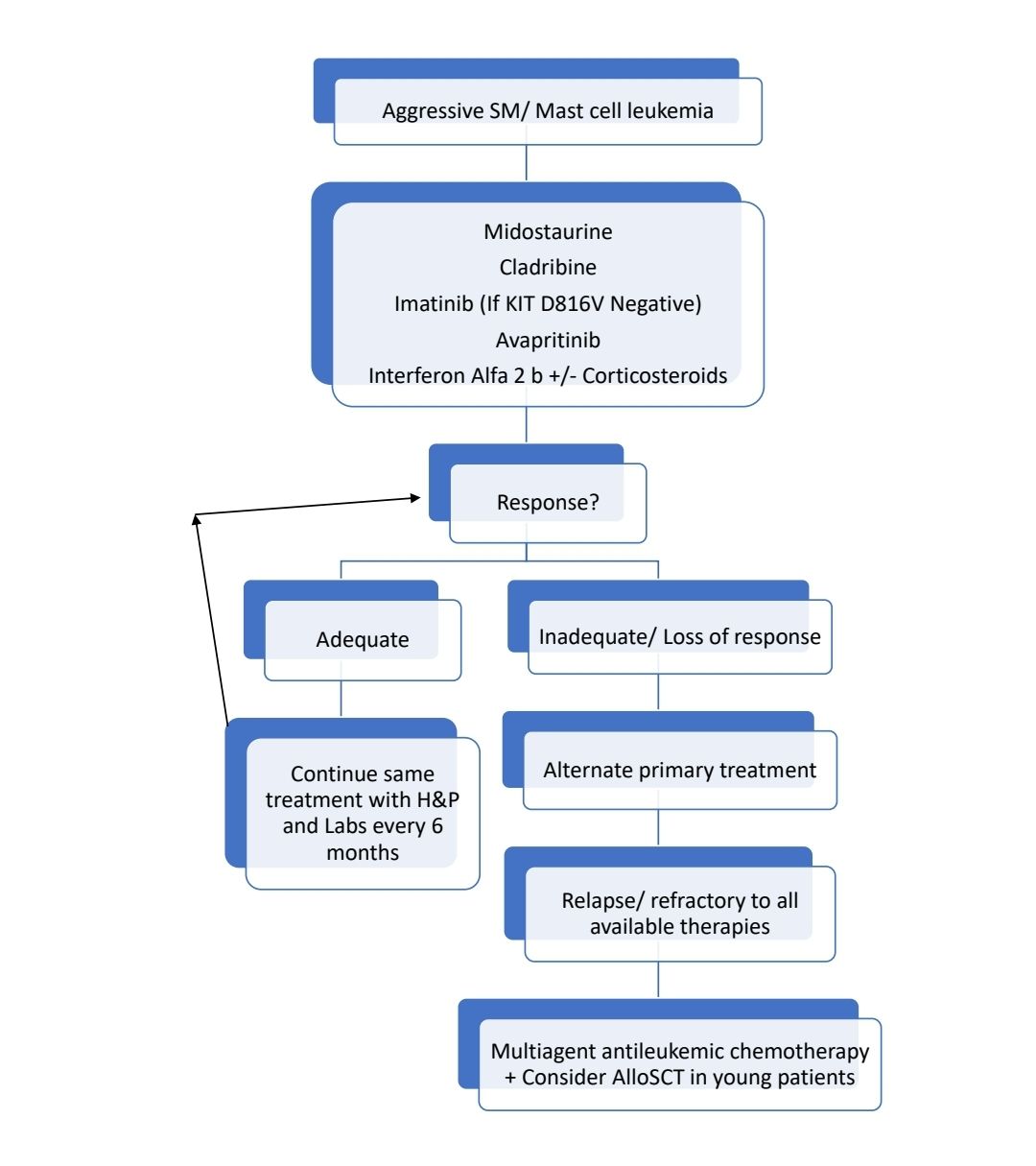
Aggressive systemic mastocytosis
- One or more “C” findings
Mast cell leukemia
- Meets criteria for systemic mastocytosis
- Biopsy shows diffuse infiltration, usually interstitial pattern, by atypical, immature mast cells
- Bone marrow aspirate smears show 20% or more mast cells
- Mast cells account of 10% or more of peripheral blood white cells
Mast cell sarcoma
- Unifocal mast cell tumor
- No evidence of systemic mastocytosis
- No skin lesions
- Destructive growth pattern
- High grade cytology
Prognosis
- Cutaneous mastocytosis of children regresses spontaneously during puberty
- There is no cure. Symptomatic therapy does not significantly alter the course of disease.
- Poor prognostic markers:
- Advanced age
- History of weight loss
- Lack of skin involvement
- Cytopenia
- Hypoalbuminemia
- Eosinophilia
- Hepatosplenomegaly
- Increased alkaline phosphatise and LDH
- Poor risk cytogenetics such as monosomy 7 and complex karyotype
- Myeloid mutation panel- Positive- SRSF2, ASXL-1, RUX1
Pretreatment Work-up:
- History: Potential triggers, Medications used, ?Cutaneous symptoms
- Examination: Liver: Spleen:
- WHO P. S.
- BSA
- IHC/Flow cytometry
- S. Tryptase levels
- BMA and Bx
- CT (CAP)/ MRI: For B and C findings
- Dexa scan
- Stage
- Haemoglobin
- TLC, DLC
- Platelet count
- LFT: Bili- T/D SGPT: SGOT: Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid:
- LDH
- HIV:
- HBsAg:
- HCV:
- UPT
- Cytogenetics
- KIT D816V testing
- FIP1L1-PDGFRA if eosinophilia present
- Myeloid mutation panel
- DEXA Scan
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
Treatment Plan:
Response criteria:
- Major Response: Complete resolution of at least one (one or more) C-finding(s) and no progression in other C-findings
- Complete remission: disappearance of mast cell infiltrates in affected organs, decrease of serum tryptase to <20 ng/mL, and disappearance of SM-associated organomegaly
- Incomplete remission: decrease in mast cell infiltrates in affected organs, and/or substantial decrease of serum tryptase level, and/or visible regression of organomegaly
- Pure clinical response: without decrease in mast cell infiltrates, without decrease in tryptase levels, and without regression of organomegaly
- Partial Response: Incomplete regression of one or more C-finding(s) a, without complete regression, and no progression in other C-findings
- Good partial response: >50% regression
- Minor response: ≤50% regression
- No Response: C-finding(s) persistent or progressive
- Stable disease: C-finding-parameters show constant range
- Progressive disease: one or more C-finding(s) show progression
About Each Modality of Treatment:
Indolent/ smoldering SM
- Preventing mast cell activation and antimediator therapy
- Avoid factors that trigger mediator release from mast cells like general anaesthesia, contrast radiography, insect stings, morphine, dextran, sudden changes in temperature, rubbing etc
- Use skin moisturizer- Water soluble sodium cromolyn cream- 2-3 times a day
- Topical corticosteroids if required
- H1 antihistaminics (Useful for pruritus and flushing)
- Cetirizine 5–10 mg/d
- Fexofenadine 60 mg BID or 180 mg/d
- Hydroxyzine 25 mg q 6 h
- Side effects: Headache, somnolence, confusion, asthenia, xerostomia
- H2 antihistaminics (Useful for abdominal pain, cramping, diarrhea, heartburn, nausea, vomiting
- Ranitidine 150 mg BID
- Famotidine 10 mg BID
- Cimetidine 400 mg BID
- Side effects: Headache, abdominal pain, dizziness, constipation, diarrhea
- Leukotriene receptor antagonist
- Montelukast 10 mg/d
- Zafirlukast 20 mg BID
- Side effects: Headache
- Aspirin-
- For skin lesions
- Side effects: Gastrointestinal bleeding, peptic ulcer disease
- Precautions: may precipitate anaphylactic reaction, children/adolescents with flu (Reye's syndrome), hepatic or renal dysfunction, bleeding disorders
- PUVA therapy
- Side effects: Nausea, pruritus, erythema of varying degree, increased risk of non-melanoma skin cancers.
- Contraindications: Pregnancy, xeroderma pigmentosa, lupus erythematosus with photosensitivity
- Disodium cromoglycate
- 100–200 mg QID 30 minutes before meals and bedtime
- Side effects: Dysgeusia, cough, osmotic diarrhea
- Systemic glucocorticoids (Prednisone)
- Dose: 0.5–1 mg/kg/d starting dose; taper as feasible based on response/ tolerance
- Other drugs which are useful
- Ketotifen
- NSAIDs
Treatment of acute mediator release/ Anaphylaxis
- IM adrenaline (Patients must carry prefilled epinephrine injections with them always)
- Can be repeated 3 times, every 5 minutes
- IV fluids
- Antihistamines (H1 and H2 blockers): diphenhydramine (25 mg every 2–4 h up to 100 mg/24 h)
- Corticosteroids: Methyl prednisolone- 125mg- Stat followed by prednisolone:0.5–1 mg/kg for 2-3 days
- Oxygen
- Corticosteroids
- Treatment of chronic mediator release- corticosteroids
- Bisphosphonates and supplemental calcium with vitamin D for osteoporosis
Treatments useful in Aggressive SM
- Midostaurine:
- Multikinase inhibitor with potent activity against both wild-type and mutant KIT.
- Dose: 100mg- BD- in continuous 28 days cycles until progression or intolerable side effects.
- ORR- 60%
- Side effects: Nausea, vomiting, diarrhea, myelosuppression
- Cladribine:
- 5mg/m2/day or 0.13 to0.17 mg/kg/day as 2 hr infusion- 5 day treatment cycle every 4 to 12 weeks up to 6 cycles.
- ORR: 55-70%
- Imatinib- May be useful in patients who lack D816V mutation of KIT gene. This KIT mutation affects catalytic pocket of KIT protein, because of which Imatinib cannot bind it.
- Avapritinib:
- Selective KIT inhibitor
- Dose: 200 mg once daily
- ORR: 57%
- Use only if platelet count >50,000/cmm
- Interferon alpha
- Pegylated forms are better tolerated
- Starting dose: 1–3 MU SQ three times per week
- Target dose: 3–5 MU SQ 3–5 times per week
- May be combined with prednisolone
- ORR: 40%
- Side effects: flu-like symptoms, bone pain, fever, cytopenias, depression, and hypothyroidism
- Allogeneic stem cell transplantation
- Useful in relapse/ refractory cases
- Overall 3 year survival: 57%
SM with associated hematological neoplasm:
- Treatment must be directed towards associated hematological Neoplasm
Monitoring After Treatment/ Follow-up:
- History and examination along with labs, once in 6 months
- Dexa scan once a year
Figures:
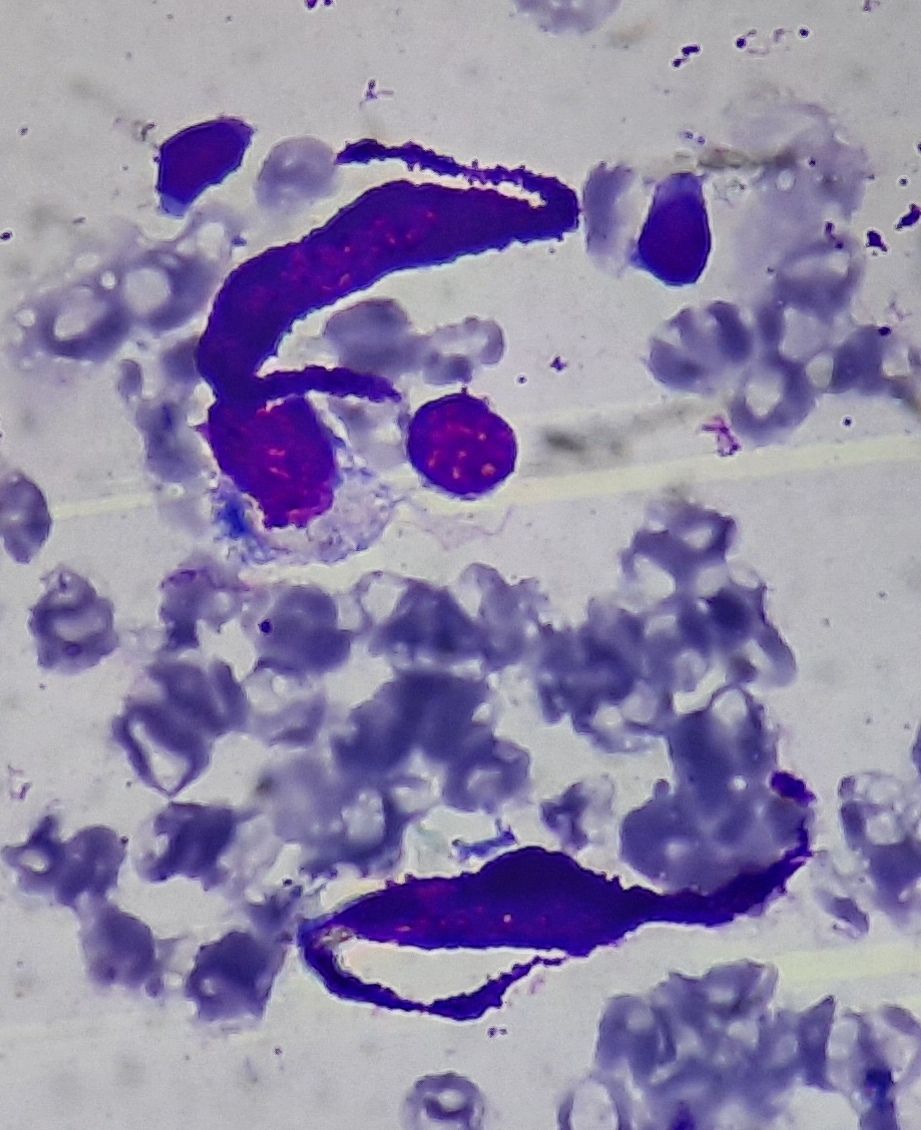
Figure 2.8.1- Mast cells in bone marrow
Recent advances:
Midostaurin in Advanced Systemic Mastocytosis
The study included data from the German Registry on Disorders of Eosinophils and Mast Cells. It was found that midostaurin is more effective compared to cladribine patients with advanced systemic mastocytosis.
https://doi.org/10.1200/JCO.21.01849
Efficacy and safety of mammalian target of rapamycin inhibitors in systemic mastocytosis
Systemic mastocytosis (SM) involves the accumulation of atypical mast cells, with advanced forms (Adv-SM) having poor survival and non-advanced forms (non-Adv-SM) often affecting quality of life despite normal life expectancy. This study evaluated the outcomes of SM patients treated with mammalian target of rapamycin inhibitors (imTOR) in France, focusing on those who were relapsing, treatment-refractory, or ineligible for other therapies. Non-Adv-SM patients received imTOR monotherapy, achieving a 60% overall response rate (ORR), while Adv-SM patients, receiving imTOR alone or with cytarabine, had a 20% ORR. These results suggest imTOR's potential benefits, particularly for those with the KIT D816V mutation.
https://doi.org/10.1002/ajh.27323
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.