howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Megaloblastic Anaemia
Introduction:
- It is an anaemia resulting from defect in nuclear maturation, primarily attributed to large degree of ineffective erythropoiesis.
Folic Acid
- Molecular weight – 441D
- Chemically – Pterygoglutamic acid
- Structure contains 3 parts Pteridine, P – amino benzoic acid and Glutamic acid
- Active form – Tetrahydrofolate (Four hydrogen reduction of pteridine ring)
- Functions – Single carbon unit transfer by N5-10 methylenetetrahydrofolate
- Uracil is converted to thymidine by addition of carbon
- Metabolism of histidine to glutamic acid, Serine to glycine, Homocysteine to methionine, Glycinamineribotide to Formylglycinamideribotide (purine)
- Source: Green leafy vegetables, fruits, beans, mushrooms, Eggs, milk, yeast and liver
- It is destroyed by heat
- Ascorbate protects folate from oxidation and heat degradation
- Present in food as conjugated polyglutamate form
- Deconjugated in intestine to monoglutamate form by a deconjugase enzyme
- Absorption mainly occurs in proximal jejunum
- In intestine it is reduced to N5 methyl tetrahydrofolate
↓
Distributed throughout the body via blood
(1/3rd is loosely bond to albumin and 2/3rd unbound)
↓
Attaches to cells by means of specific receptors
↓
Inside cells it is demethylated and conjugated to avoid leaking out again
(This needs cobalamin, so in B -12 deficiency cells are unable to retain folate
Hence in case of Vitamin B 12 deficiency , megaloblastic anemia is due to intracellular folate deficiency)
- RDA: - 400 µg
- 50 – 80% is absorbed
- Daily requirement – 50 µg / day
- Liver stores – 5 – 10mg (Enough to provide folate of 3 -6 months)
- During pregnancy, 600µg is required per day. Deficiency is associated with neural tube defects.
Cobalamin (Vitamin B–12)
- Structure – Corrinoid structure
- Corrin ring- Formed by joining 4 reduced pyrrolesubrings which are linked to central cobalt.
- Nucleotide - Lies perpendicular to the ring and attached to the ring and to cobalt.
- β group – Attached to cobalamin on opposite side of the ring from the nucleotide. It can be cyanide, methyl, adenosyl or hydroxyl
- Source:
- Animal origin – Fish, milk, Eggs and meat (liver & kidney- 100µg/100g)
- Vegetables are free from cobalamin unless they are contaminated by bacteria
- B12 producing bacteria (Kl. Pneumoniae etc) can be found in small intestine of some individuals from which cobalamin can be absorbed.
- Requirement– 5 microg/ day
- 70% of ingested B 12 is absorbed, so RDA is – 5 - 7 microg/day
- Stores – 5000 microg (sufficient for 1000 days i.e., 3years)- Stored in liver
- Absorption
- Passive
- Occurs in duodenum & ileum
- Extremely inefficient
- < 1% of oral dose in absorbed through this route
- Active:
- Passive
Dietary cobalamin is released from protein complexes by enzymes in stomach, duodenum & jejunum
↓
Binds rapidly with salivary glycoprotein – R – binder (haptocorrin)
↓
R- binder is digested by pancreatic trypsin
↓
B – 12 binds to intrinsic factor (which is favored by alkaline pH)
(Intrinsic factor- cobalamin complex is resistant to enzyme digestion)
↓
Intrinsic factor – B 12 complex binds to cubulin on epithelium of ileum
↓
Cobalamin enters the cell
↓
Cubulin is sent back to brush border to carry more vitamin B12
(As there are limited number of cubulin, intestinal absorption of vitamin B12 cannot be increased to a greater extent by increasing the dose)
↓
Vitamin B12 is processed and sent to portal circulation
↓
Holotranscobalamin is formed in liver by combination of vitamin B12 with transcobalamin 2
↓
Holotranscobalamin is released into systemic circulation
↓
Binding of holotranscobalamin to transmembrane transcobalamin II receptor
↓
Holotranscobalamin enters cell by process of endocytosis
↓
Lysosomal degradation and release of cobalamin for metabolic functions
- 90% of body stores of vitamin B12 is found in liver
- To be useful to cell hydroxy/cyanocobalamin should be converted to adenosyl/ methyl cobalamin
- Adenosyl cobalamin is required for
- Conversion of methyl malonyl CoA to succinyl CoA (which is mediated through enzyme methylmalonyl Coa Mutase)
- Methylation of homocysteine to form methionine, which is linked to folate metabolism
Lack of Vitamin B12 causes accumulation of Methyl malonyl CoA
↓
Accumulation of PropionylCoA
↓
Synthesis of certain fatty acids with odd number of carbons from PropionylCoA instead of acetyl CoA
↓
These abnormal fatty acids are incorporated into neuronal lipids.
↓
Disruption of membrane functions.
↓
Neurological complications like demyelination.
Causes of Vitamin B-12 Deficiency
- Inadequate content in diet –
- Absence of meat in diet (Strict vegans)
- Decreased absorption
- Pernicious anemia (Responsible for 85% of cases of Vitamin B-12 deficiency. Characterised by antibodies against intrinsic factor)
- H. Pylori infection
- Total gastrectomy (Lack of intrinsic factor)
- Malabsorption syndrome
- Blind loop syndrome (Competitive uptake of B12 by bacteria)
- Ileal resection (More than 1.2mts)
- Diffuse intestinal disease
- Competitive parasitic uptake especially Diphyllobothrium latum.
- HIV (As transcobalamin II is derived from macrophages)
- Zollinger Ellison syndrome (Failure to release cobalamin from R – binder due to inactivation of pancreatic trypsin by high acidity)
- Congenital vitamin B – 12 malabsorption
- Congenital intrinsic factor deficiency (Autosomal recessive)
- Inherited transcobalamin II deficiency (Autosomal recessive, treated with large doses of Vitamin B12, to push it into the cell- 1000microgm- IM- Weekly- lifelong)
- Imerslund – Grasbeck disease (Autosomal recessive condition, mutation in cubulin receptor gene, Associated with proteinuria)
- Drugs – phenformin, metformin, PAS, Cholestyramine, Cimetidine, neomycin, colchicine, proton pump inhibitors
- Increased demand
- Pregnancy
- Hemolytic anemia, Sideroblastic anemia
- Myeloproliferative disorders, leukemia, lymphoma
- Carcinomas
- Hyperthyroidism
- Nitrous oxide anaesthetic inhalation (Destruction of endogenous cobalamin by oxidation, resulting in acute megaloblastic anemia, which presents with rapidly developing thrombocytopenia and leukopenia)
Causes of Folic acid deficiency
- Decreased intake
- Inadequate content in diet (Folate is destroyed by cooking)
- Infancy –institutional diet, Goat’s milk
- Decreased absorption
- Malabsorption syndrome.
- Diffuse intestinal diseases- Celiac disease, tropical sprue, Crohn’s disease
- Alcohol ingestion especially whisky
- Drugs – Phenytoin, phenobarbitone, OC pills, anticoagulants, primidone, sulfasalazine, cholestyramine etc.
- Congenital folate malabsorption (Mutation in SLC46A1 gene which causes abnormality of proton coupled folate transporter, Presents with seizures and mental retardation, Rx- Daily folinic acid injection)
- Increased loss
- Haemodialysis- As folate is loosely bound to plasma proteins, it is easily removed by dialysis
- Increased requirement
- Pregnancy (Need 400 micro g/day), puberty, lactation
- Prematurity
- Hemolytic anemia – sickle cell anemia, thalassemia, spherocytosis.
- Myeloproliferative disorders, leukemias.
- Metastatic cancer.
- Inflammatory diseases- Tuberculosis, Crohn’s, rheumatoid arthritis, psoriasis, malaria etc.
- Metabolic diseases- Homocystinuria.
- Excess of urinary loss- CCF, active liver disease (Due to release of folate from damaged liver cells)
- Hypothyroidism.
Drugs that impair DNA metabolism
- Purine antagonist- 6-mercapto purine, 6- thioguanine, Azathioprine, Methotrexate, vidarabine
- Pyrimidine antagonist- 5 fluorouracil, cytosine arabinoside
- Others- Procarbazine, Hydroxyurea, acyclovir, gancyclovir, Zidovudine, pentamidine, Trempterine, Trimethoprine, Primidone, cyclophosphamide, Azacytidine, pyrimethamine, phenytoin, phenobarbital, carbamazepine, OC pills, proton pump inhibitors, Metformin
Metabolic diseases.
- Hereditary oroticaciduria (Defect in pyrimidine metabolism)
- Lesch –Nehan syndrome
- Congenital deficiency of folate metabolizing enzymes- Dihydrofolatereductase deficiency, Methylenetetrahydrofolatereductase deficiency
- Thiamine responsive megaloblastic anemia- Defect high affinity thiamine transport gene. Associated with nerve deafness & diabetes mellitus. Treatment- Thiamine- 25-100mg/day
- Methioninesynthase deficiency.
- Congenital dyserythropoietic anemia
- Homocysteinuria& methyl malonylaciduria
Pathogenesis:
Decreased dTMP and increased dUTP
↓
dUTP is incorporated into DNA of folate deficient cells
↓
Failure of DNA excision repair mechanism due to unavailability of dTMP
↓
Impaired DNA synthesis, DNA strand breaks
↓
Nucleus instead of becoming pyknotic remains relatively large and immature.
(Disassociation between nuclear and cytoplasmic maturation)
↓
Accumulation of megaloblasts in bone marrow
↓
Ineffective erythropoiesis, as many cells die in premitotic phase due to apoptosis.
↓
Decreased yield of RBCs
↓
Anemia
Ineffective erythropoiesis also results in
- Unconjugatedhyperbilirubinemia
- Raised urobilinogen
- Reduced haptoglobins
- Positive urine hemosiderin
- Elevated LDH (> 1000 – 10,000 IU/dL)
- Elevated serum iron and ferritin levels
Clinical Features:
- Anemia- Pallor with lemon tint icterus
- Fever in case of severe anemia
- Anorexia
- Weight loss
- Failure to thrive
- Diarrhoea/ constipation
- Atrophic glossitis – Shiny, glazed, beefy tongue, angular cheilosis (as deficiency affects DNA synthesis in all rapidly multiplying cells)
- Jaundice- Unconjugatedhyperbilirubinemia.
- Knuckle pigmentation and reversible melanin skin pigmentation
- Bruising (due to thrombocytopenia)
- Increased risk of infection (due to low WBC counts)
- Infertility (as gonads are affected)
- Neuropsychiatric symptoms (seen with cobalamin deficiency)- Depression, irritability, forgetfulness, sleep deprivation, cognitive dysfunction, paranoid ideation, dementia, delusions
- Neurological manifestations- Occur due to cobalamin deficiency (Sometimes occurs without anemia or macrocytosis but BM shows megaloblastic changes. These manifestations get aggravated with administration of folic acid without vitamin B12 supplementation)
- Peripheral neuropathy: Paraesthesia, muscle weakness difficultly in walking, Burning sensation of tongue, Vague abdominal pain
- Demyelination of posterior & pyramidal tracts of spinal cord (Subacute combined degeneration)- Loss of vibration sense for 256HZ but not for 128Hz, Extensor plantars, Spastic paraplegia, sensory ataxia, severe paraesthesia in lower limbs, Sphincter disturbance, Decreased reflexes, Romberg’s and Babinski’s sign- Positive, Loss of position sense in second toe
- Optic atrophy & retro bulbar neuritis- Seen especially in patient who smoke.
- Nonhematological effects of folate deficiency
- Arterial thrombosis due to increased homocysteine (This role is uncertain)
- Syndrome similar to HELLP- Differentiated by presence of anemia and macrocytosis
- Colon cancer- Supplementation of folate decreases risk of colon cancer by 31% (Folate helps in DNA repair. But if already tumour cells are formed, folate helps in their proliferation)
Investigations:
- Hemogram:
- Hemoglobin –It is less than 12gm/dl, only in 29% cases.
- MCV-Elevated to more than 100fL
- MCH-Elevated
- MCHC- Normal
- Macrocytic anemia, ovalocytosis (Co-existing iron deficiency/ thalassemia/ inflammation may prevent macrocytosis)
- Poikilocytosis if anemia is severe
- Normo / hyperchromasia
- Increased polychromatophilic cells
- Many nucleated RBCs may be seen resulting in leucoerythroblastic reaction
- Howell Jolly bodies and Cabot’s rings may be seen
- Leucopenia due to absolute neutropenia (but usually not less than 1,500/cmm)
- Neutrophils are larger and have hypersegmented nuclei
- When more than 5% of neutrophils have more than 5 lobes or at least one 6 lobed WBC in a film is present, it is called hypersegmentation.
- Polymacrocyte is a large polymorph with nucleus containing up to 9 lobes
- Hypersegmentation may also be found in case of renal failure, congenital form and in iron deficiency states.
- Platelet count is moderately reduced (but not less than 40 x 109 /Lit)
- Large forms of platelets are seen especially when counts are markedly reduced.
- Bone marrow aspiration
- Generally, not required if biochemical parameters are consistent with megaloblastic anemia.
- Cellularity is increased (Rarely hypocellular)
- Myeloid to erythroid ratio is 1:1
- Erythroid precursors show megaloblastic changes
- Cells are much larger than erythroblasts
- Nucleus has fine, reticular chromatin which gives a stripped appearance (Loose open/ salt pepper granular chromatin) and large nucleoli.
- Nucleocytoplasmic asynchrony- Maturation of nucleus lags behind that of cytoplasm (Nucleus is young and cytoplasm is mature).
- Fully hemoglobinized orthochromatic erythroblasts which retain nuclei may be seen.
- Howell-Jolly bodies (Fragmented nuclei) may be seen.
- Abnormal and more common mitosis.
- Maturation arrest is visible.
- These changes disappear after 24hours of administration of vitamin B12 and folic acid supplementation
- Megaloblastic marrow may also be seen in case of iron deficiency anemia, sideroblastic anemia, erythroleukemia, chronic anemia thalassemia, chromic infection etc
- Masked megaloblastosis: Classic findings of megaloblastic changes may not be seen due to co-existing conditions the neutralize the tendency to generate megaloblastic cells. Ex: Iron deficiency anemia, thalassemia, anemia of chronic disease. There is a wide RDW in these conditions.
- Increased iron granules on Prussian blue staining.
- Leucopoiesis
- Giant metamyelocytes (Giant stab) and bands with large horseshoe shaped nuclei containing loose, open chromatin in nuclei are diagnostic
- Myelocytes show poor granulation
- Large atypical granulocytes may be seen.
- Megakaryocytes are abnormally large with multilobulated (hyperdiploid) nucleus and deeply basophilic agranular cytoplasm.
- Microscopy of other cells: Epithelial cells of stomach, mouth and cervix may look megaloblastic. These cells appear larger than their normal counterparts and contain atypical immature looking nuclei. Distinguishing these "megaloblastic changes" from changes of malignancy can be difficult.
- Reticulocyte count- Normal, but RPI is less than 2
- Serum LDH level- Levels of fraction 1and 2 are markedly elevated. This is due to destruction of megaloblasts which are rich in LDH
- Serum levels of vitamin B-12-
- Decreased to less than 200pg/ml(Normal-200-350pg/ml)
- It can be normal in clinically proven megaloblastic anemia
- Holotranscobalamin assay (In this Vitamin B-12 bound to transcobalamine is measured). It represents 20-30% of total vitamin B-12
- Serum folate assay:
- Normal -3-25ng/ml
- Rapid transport of sample is important, as folate is unstable and is destroyed by heat and light. (Adding sodium ascorbate stabilizes folate. But this sample cannot be used for assessment of vitamin B12.)
- Red cell folate assay
- Red cells contain 20-25 times more folate than in serum
- This assay is more accurate, as serum levels fluctuate with dietary intake.
- It is not useful in cobalamin deficiency, as in this case RBC folate is reduced.
- Normal -340-640 ng/L of packed cells
- Intrinsic factor antibody test: Positive in pernicious anemia
- Schilling test
- Radioactive vitamin B-12 is given orally followed 2hours later by high dose of parenteral B-12 injection.
- Normally more than 10% of administered radioactive Vitamin B-12 is excreted in urine as large amount of parenteral unlabelled B-12 binds to most tissue binding sites.
- Patients with malabsorption of Vitamin B-12 excrete <5% of administered radioactive-B-12
- In such patients radioactive B-12 is again given orally along with intrinsic factor
- If urinary excretion of radioactive Vitamin-B12 now rises to >10% it indicates patient is suffering from pernicious anemia.
- Presently this test are not done due to
- Reduced availability of test components
- High cost
- Problems with radioactive waste disposal
- Concerns about use of animal derived tissues for human use (Intrinsic factor)
- FIGLU excretion test
- Folate is required for conversion of FIGLU (which is formed from histidine) to glutamic acid.
- Large dose of histidine is given to the patient.
- Increased excretion of FIGLU indicates folate deficiency
- Serum gastrin level- Increased in pernicious anemia
- Serum ferritin and iron levels- Increased due to large degree of ineffective erythropoiesis
- Haptoglobins, Uric acid and alkaline phosphatase levels – Decreased
- Serum methylmalonate and homocystein levels
- Vitamin B-12 is needed for conversion of methylmalonate to SuccinylCoA and homocystein to methionine
- So in Vitamin -B-12 deficiency methylmalonate and homocysteine levels are raised.
- Other conditions in which homocysteine level is raised.
- Chronic renal disease.
- Small bowel bacterial overgrowth
- Haemoconcentration
- Alcoholism.
- Smoking.
- Pyridoxine deficiency.
- Hypothyroidism.
- Drugs – steroids, cyclosporine etc
- Gastric biopsy- For pernicious anemia
- Serum antiparietal cell antibody assay
- Small intestinal studies- Duodenal biopsy to rule out celiac disease
- Stool for fish tapeworm ova.
Pretreatment Work-up:
- History
- Examination
- Hemoglobin
- MCV
- TLC, DLC
- Platelet count
- Peripheral smear
- Reticulocyte count
- S. B12 Levels
- S. Folic acid level
- Anti- IF antibody
- LFTL Bili- T/D SGPT: SGOT:Albumin: Globulin:
- Creatinine
- LDH
Treatment:
- Treat severely ill patient with both vitamins in large doses, once the blood sample has been taken for vitamin B-12 and folate assay.
- Try to identify the cause by history, examination and investigations. If any cause is found treat it promptly.
- Transfusion is not necessary, but if needed packed red cells should be given. If decompensated transfuse under cover of furosemide.
- Platelet concentration should be given if there is spontaneous bleeding
- Vitamin B-12 (Hydroxo/ cyanocobalamin)-
- 1000 micrograms – IM/IV
- Daily for 5-7 days
- Then- weekly for 5 weeks
- Then- once in 3 months (If neurological deficits at presentation- once in 2 months)- Lifelong
- Transient hypokalemia is often seen- Treat with oral potassium supplements
- Reticulocytosis peaks at 1 week and becomes normal by 20th day.
- Hemoglobin returns to normal in 5-6 weeks.
- LDH falls to 50% in 72 hrs.
- Normal pronormoblasts appear within 4-6 hours, and complete recovery of erythroid abnormalities occur within 2-4 days.
- Hypersegmented neutrophils disappear after 15 days.
- Peripheral neuropathy may be reversed, but spinal cord damage is irreversible.
- In case of vitamin B-12 deficiency if folate alone is given it aggravates neurological damage. Hence avoid multivitamin preparations
- Oral supplements are available and are useful even in cases of pernicious anemia. Dose- 1,000-2,000 micrograms/day
- 1000 micrograms – IM/IV
- Folic acid
- 5mg/day-orally
- Continue the therapy for 4 months, till all folate deficient RBCs are eliminated.
- Treatment of causes:
- Diphyllobothriumlatum: T. Praziquantel 5-10mg/kg- Single dose
- Pyrimethamine, trimethoprime and most of other drugs- Folinic acid
- Congenital dihidrofolatereductase deficiency- Folinic acid
- Blind loop syndrome: Broad spectrum antibiotics (Cephalexin 250mg QID + Metronidazole 250mg- TID) for 2 weeks, Surgical correction of anatomical lesion
Causes for non-response to therapy
- Wrong diagnosis
- Combined folate and cobalamine deficiency being treated with only one vitamin
- Associated iron deficiency
- Associated hemoglobinopathy. Ex. Sickle cell disease, thalassemia
- Associated anemia of chronic disease
- Associated hypothyroidism
Indications for prophylaxis
- Cobalamin:
- Infants on specialized diets- On vegetarian diet- PO. 5-10microgam/day, if malabsorption- PO- 1000microgm/day (Absorption happens by passive diffusion)
- Premature infants
- Infants of mothers with pernicious anemia
- Infants and children of mothers with nutritional cobalamin deficiency
- Vegetarians and poverty induced near vegetarianism
- Total gastrectomy
- Folic acid:(Dose- 400micrograms/day)
- All women contemplating pregnancy
- Pregnancy and lactation
- Premature infants
- Mothers at risk of delivering infants with neural tube defects (Previous history of delivery of child with NTD. Dose- 5mg/day)
- Patients with rhematoid arthritis/ psoriasis on therapy with methotrexate(Dose- 5mg/day)
- Hemolytic anemia/ hyperproliferativehematological states (Dose- 5mg/day)
- Patients of ulcerative colitis
- Patients on antiepileptic drugs
Special Situations:
- Maternal folate deficiency
- Results in
- Prematurity
- Recurrent fetal loss
- Childhood behavioural abnormalities
- Learning problems
- Neural tube defects (Anencephaly, meningomyelocele, encephalocele, spina bifida).
- Incidence in India- 9 in 1000 live births
- Prevented by 0.4mg folic acid daily.
- Neural tube folding occurs by 28 days, before even woman knows that she is pregnant. Hence better to give folate supplements to all those who are planning pregnancy.
- Prophylaxis during pregnancy also prevents acute lymphoblastic leukemia in children
- Results in
Related Disorders:
- Pernicious anemia
- It is commonly seen after 40 years of age.
- It is characterized by autoimmune destruction of gastric mucosa leading to chronic atrophic gastritis.
- 3- Types of auto antibodies are found (IgG type).
| Type | Character | Prevalence |
1. | Blocking Ab | Blocks vitamin B-12 and IF binding | 75% |
2. | Binding Ab | Reacts with IF and prevents its attachment to ileal mucosa | 50% |
3. | Parietal CanalicularAb | Directed against α and β subunits of gastric proton. | 90% |
- Risk Factors
- ?Genetic- More common in people with blood group A.
- Other autoimmune disease – Grave’s disease, Hashimoto thyroiditis etc
- Association with HLA-B8, B12, BW15.
- ?H. Pylori triggered autoimmune reaction
- These patients are 3 times more prone to develop carcinoma of stomach
- Investigations
- Gastric biopsy
- Loss of parietal cells and chief cells.
- Prominent infiltration of lymphocytes and plasma cells.
- Changes are marked in body of stomach
- Metaplasia of gastric epithelium to intestinal type
- Serum gastrin levels- Elevated
- Serum Somatostatin levels- Decreased
- Serumpepsinogen I levels-Decreased
- Gastric function test-Histamine unresponsive achlorhydria
- Serum antiparietal cells antibodies
- They may be present also in cases of gastritis, thyroid disease and Addison’s disease
- Gastric biopsy
- Treatment
- Parenteral vitamin B-12 therapy
- Follow up for early detection of gastric carcinoma
- Steroids can be used but it is associated with increased risk of malignancy and improvement in gastric lesion is only temporary.
- Juvenile pernicious anemia
- It is a familiar condition, characterized by congenital deficiency of intrinsic factor.
- It is commonly associated with endocrinopathies.
- Infantile onset cerebral folate deficiency:
- Develops 4-6 months after birth
- Presents with agitation, insomnia, delayed development of head, psychomotor retardation, cerebellar ataxia, pyramidal tract signs in legs, dyskinesias, severe polyneuropathy, sometimes seizures
- Pathogenesis: Infants fed on cow's milk develop autoantibodies to milk folate binding proteins. These antibodies bind to folate receptors in choroid plexus and block thefolate receptor mediated folate transport across the cerebrospinal fluid. This leads to cerebral folate deficiency
- Investigations:
- S. Folate: Normal
- CSF Folate- Reduced
- S. Folate receptor auto antibodies- Present
- Treatment
- High dose folinic acid
- Bovine milk free diet
Figures:
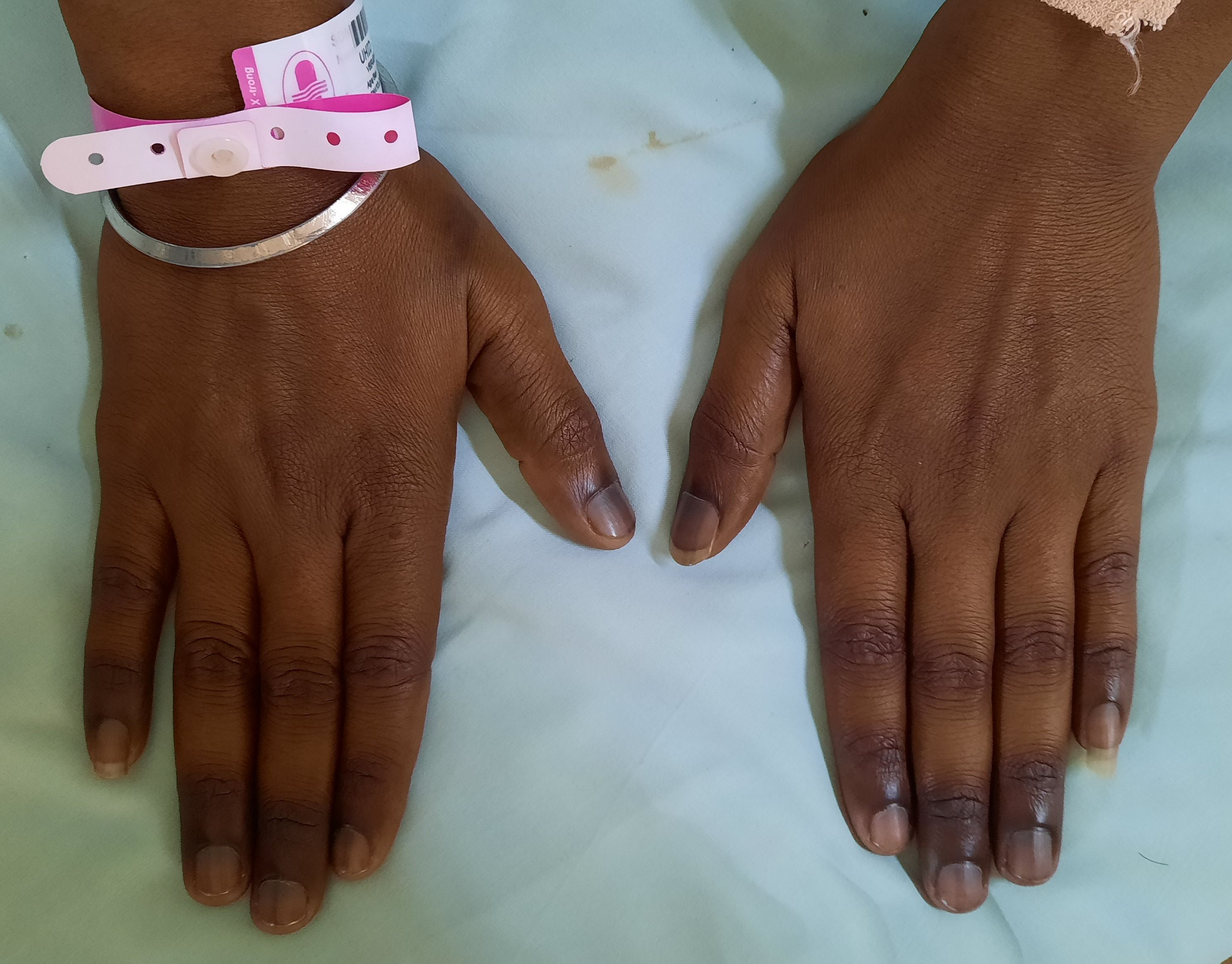
Figure 8.2.1- Knuckle pigmentation
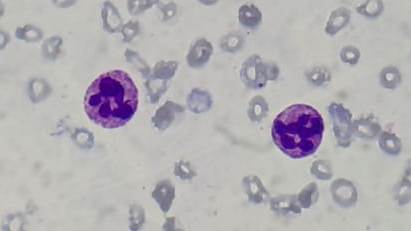
Figure 8.2.2- Megaloblastic anemia- Peripheral smear- Hypersegmented neutrophils
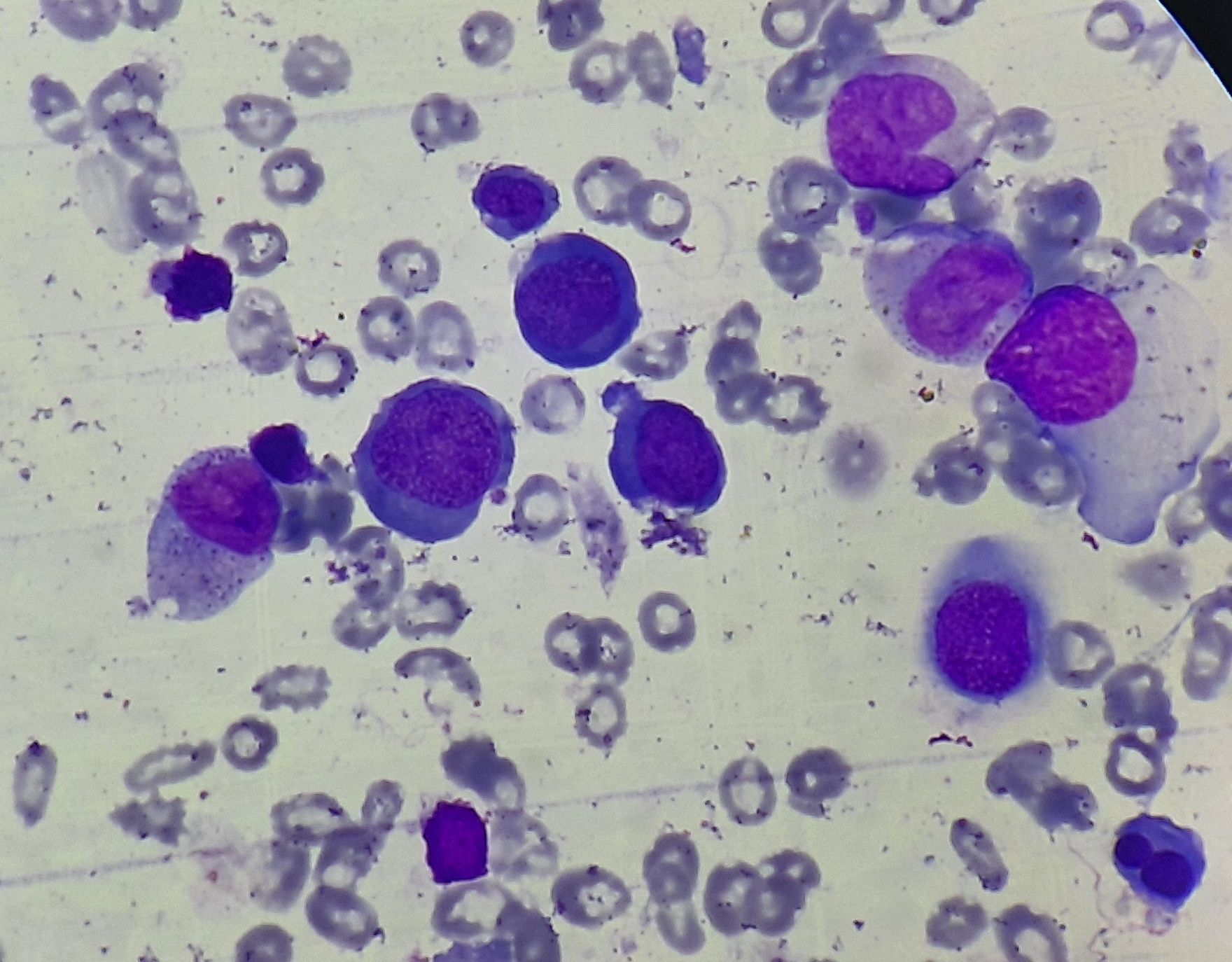
Figure 8.2.3- Megaloblastic anemia- Bone marrow aspiration
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.