howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Plasma Cell Neoplasms
This category includes:
- Plasma Cell Myeloma
- Smouldering (asymptomatic) myeloma
- Non-secretory myeloma
- Plasma cell leukemia
- Plasmacytoma
- Solitary Plasmacytoma of Bone
- Extramedullary Plasmacytoma
- Plasma cell neoplasms with associated paraneoplastic syndrome
- POEMS Syndrome
- TEMPI Syndrome
- AESOP Syndrome
- Monoclonal gammopathy of unknown significance
- Monoclonal gammopathy of clinical significance
- Heavy chain diseases
- Idiopathic Capillary Leak Syndrome
Plasma cell Myeloma
(Multiple myeloma)
Introduction:
- It is a bone marrow based, multifocal plasma cell neoplasm characterized by elevated serum monoclonal proteins, skeletal destruction, hypercalcemia and anemia.
Epidemiology:
- It accounts for 1-2% of all malignancies
- In US it is most common lymphoid malignancy in blacks and second most common in whites.
- Men are commonly affected than women. (M:F- 1.4:1)
- It represents 15% of all hematological malignancies
- Median age- 70 years
- 3-4 cases per 1lac population
Etiology: Exact cause is not known
- Environmental agents – pesticides, petroleum products, asbestos, rubber, plastic and wood products, dioxins, solvents, cleaners etc.
- High dose irradiation (100 cGy)
- Chronic inflammation (chronic antigenic stimulation) such as osteomyelitis, rheumatoid arthritis, HHV-8 infections, EBV, HIV, hepatitis virus infection, infection with “Stealth adapted” viruses such as mutated CMV.
- It is increasingly seen in cosmetologists, farmers and laxative users.
Pathogenesis:
- 2 hit hypothesis
- 1st hit – Initial antigenic stimulation which leads to multiple benign clones
- 2nd hit – Mutational change which leads to malignant transformation
- Bone marrow microenvironment in myeloma
- Homing in of plasma cells in BM involves
- Selective adhesion to BM endothelial cells with expression of CXCR4, CXCR3, CCR1, CCR2, CCR5
- Trans endothelial migration
- Adhesion to stromal cells through the production of stromal derived factor1 (SDF1) and insulin like GF1 (ILGF1)
- Adhesion of myeloma cells to BM stromal cells through a4 b1 integrin / VCAM1 induces production of several cytokines (mostly by NF-KB pathway)
- IL6 - Triggers proliferation of myeloma cells
- IL1b, TNF-alfa, TGF-beta - Enhance osteoclastogenesis
- RANKL- Binds to RANK on osteoclasts leading to enhanced differentiation, proliferation and survival of osteoclasts. (normally osteoprotegerin binds to RANK, but in myeloma its level is decreased)
- Some cytokines are directly produced by plasma cells
- VEGF and Beta FGF cause enhance angiogenesis
- MIP 1alfa (RANTES family) activates osteoclasts
- dickkop -1 protein which leads to inhibition of Wnt- mediated osteoblast differentiation and reduced bone formation
- Growth signals for myeloma cells are mediated through following pathways
- PITK/AKT
- STAT3
- RAS/MAPK
- NFk-beta
- Homing in of plasma cells in BM involves
- Myeloma cells may secrete immunoglobulins (Paraproteins)- They get deposited in kidney and cause renal damage
- Single heavy and light chain – known as monoclonal protein / paraprotein
- Only light chains – In urine they are known as Bence Jones proteins
- IgG – 60%
- IgA – 20%
- Light chain only - 15-20% (Risk of renal disease is high)
- IgD or IgE – 1%
- None (Non secreting) – 1% (Normal Serum, urine immunofixation electrophoresis and normal FLC ratio. M protein can be detected by IHC)
- Oligosecretory- Serum M Protein <1g/dL and Urine M Protein <200mg/24hrs
Clinical Features:
- 20% are asymptomatic
- Weight loss, malaise and fatigue
- Bone pain
- Especially in ribs and back (Due to osteoporosis, lytic lesions and pathological fractures)
- Even with adequate therapy, lytic lesions remain and new bone formation does not occur.
- Hypercalcemia
- It may be due to hyperactivity of osteoclasts and suppression of osteoblasts.
- May cause anorexia, vomiting, diarrhea / constipation, polydipsia, polyuria, muscle weakness, shortening of QT etc.
- Repeated infection (URTI, LRTI, UTI):
- Occurs due to
- Decreased protective immunoglobulins
- Neutropenia
- Hypoventilation due to pathological fractures
- Unproductive Th2 cell response due to cytokines such as IL6, TGF beta, IL 10 and beta 2 microglobulin
- Side effect of antimyeloma treatment (Cytotoxic drugs and glucocorticoids)
- Infection with encapsulated organisms such as streptococcus pneumonia, Haemophilus influenza etc are common
- Occurs due to
- Anemia:
- Seen in 75% of patients.
- Occur due to
- Myelophthiasis – Replacement of marrow by tumor cells
- Tumor production of inhibitory factors and auto antibodies (IL6 stimulates Hepcidin release)
- Expanded plasma volume due to increased paraproteins
- Renal failure- Decreased EPO production
- Chronic infection
- Bleeding
- Nutritional deficiency
- Renal failure
- Seen in 25% of patients at presentation
- 30-50% patients have some degree of renal impairment and 10% need hemodialysis
- It occurs due to
- Light chain nephropathy: Tubular damage by excretion of light chains (Lambda chains are more nephrotoxic)
- Cast nephropathy: Tubular casts block distal convoluted tubule leading to interstitial nephritis
- Hypercalcemia resulting in nephrocalcinosis
- Glomerular deposition of amyloid leading to nephrotic syndrome
- Hyperuricemia resulting in urate nephropathy
- Recurrent urinary infections
- Infiltration of kidney by myeloma cells
- Use of NSAIDs for pain control
- Nephrotoxic antibiotics and imaging contrast agents and use of bisphosphonates.
- Hyperviscosity
- May present as headache, confusion, breathlessness, visual disturbance and bleeding.
- Normal relative serum viscosity is 1.8 mpascals, and symptoms occur at 5-6 mpascals
- Common with IgA and IgG3 type paraproteinemia
- Fundoscopy: Retinal vein distension, hemorrhage and papilledema
- Amyloid deposition (AL type amyloidosis)
- May manifest as – Carpal tunnel syndrome, macroglossia, nephrotic syndrome, cardiac failure, peripheral neuropathy, subcutaneous nodules, hepatosplenomegaly etc.
- Bleeding manifestations due to
- Paraprotein causes abnormal platelet function
- Thrombocytopenia in advanced disease
- Paraprotein may act as anticoagulant by inhibition of fibrin monomer polymerization.
- Hyperviscosity
- Perivascularamyloidosis
- Acquired states such as factor 10 deficiency
- Tumor formation especially in ribs
- They are firm, tender and are associated with egg shell crackling
- Extramedullaryplasmacytoma
- Seen in 7% of patients at diagnosis
- Cord compression- Sensory loss, paresthesia, limb weakness, walking difficulty, sphincter disturbance leading to incontinence
- Neuropathy due to
- Regional myeloma cell growth compressing spinal cord or cranial nerves
- Perineural or perivasa nervosum amyloid deposition
- Therapeutic interventions (thalidomide, bortezomib, vincristine)
- Other protein deposits
- Metabolic causes related to hypercalcemia or hyperviscosity
- IgM related neuropathy- Antibodies to myeline associated globulin
- Thrombosis : Due to
- Hyperviscosity
- Defective fibrin structure and fibrinolysis because of increased IgG levels
- Increased acquired protein C resistance
- Increased pro-inflammatory cytokines such as IL6
- Sometimes lupus anticoagulants
- Thalidomide, lenalidomide and EPO use
Investigations:
- Hemogram:
- Normocyticnormochromic anemia
- Marked red cell rouleaux formation
- Abnormally blue stained background of blood film due to presence of paraproteins
- Moderate leucopenia
- Few atypical plasma cells may be present
- Sometimes there can be leucoerythroblastic blood picture
- Thrombocytopenia is rare and late, as IL6 stimulates megakaryopoiesis
- Thrombocytosis may indicate hyposplenism because of amyloidosis of spleen.
- BM aspiration
- If possible, should be done from radiologically abnormal site
- Hypercellular marrow
- Erythropoiesis and myelopoiesis are generally suppressed
- Megakaryocytes are generally adequate
- Plasma cells are increased in number
- >10% Clonal plasma cells is a major criteria for diagnosis (In aspirate/ biopsy). Clonality can be assessed by flow cytometry/ IHC.
- Diagnosis of MM can be confirmed with less than 10% plasma cells in BM, if other diagnostic criteria are fulfilled and if there is histopathological confirmation of a soft tissue or bony plasmacytoma. If disease is patchy, some patients need BM aspiration from 2-3 different sites/ imaging guided biopsy.
- They can be mature/ immature/ pleomorphic/ anaplastic (Greipp plasma cell grading system)
- Mature type- Dense chromatin clumping (“Spoke wheel” or “Clock face” chromatin), nucleus <8microns, Nucleolus- <1 micron, Cytoplasm well developed, nucleus is eccentric with prominent perinuclearhof .
- Immature type- Diffuse chromatin pattern, nucleus >10 microns, Nucleolus >2micron, abundant cytoplasm, nucleus is eccentric with hof
- Plasmablastic type- Very high N:C ratio, scanty cytoplasm, central immature large nucleus with reticular chromatin pattern, prominent nucleolus, little/ no hof
- Intermediate type- Not meeting criteria of other types
- Nuclear immaturity and pleomorphism are rarely seen in reactive plasma cells. Hence they are reliable indicators of neoplastic plasma cells.
- Morphologically distinctive varieties may be seen due to crystallized cytoplasmic immunoglobulin in endoplasmic reticulum
- Mott / morula cells- Multiple, pale, bluish – white, grape like accumulation
- Russel bodies- Cherry red refractile round bodies.
- Flame cells- Vermilion staining glycogen rich IgA
- Goucher like cells (Thesaurocytes)- Over stuffed fibrils.
- Dutcher bodies- Nuclear accumulation of immunoglobulins
- Number counted on morphology is considered gold standard, as on flow, there can be loss of plasma cells during processing or they may nor express required antigens.
- Considerable site to site variation in plasma cell density is present (Macrofocal myeloma- Multiple plasmacytoma with normal bone marrow aspiration and biopsy)
- Bone marrow biopsy
- Excess of marrow plasma cells
- Normally, plasma cells are present in small clusters of 5/6 cells around marrow arterioles
- In myeloma, they occur in large foci, nodules / sheets, and later displaces normal marrow elements.
- IHC on biopsy gives higher percentage of plasma cells compared to BM aspiration, due to relative difference in "aspirability" between plasma cells and other marrow cells.
- IHC on biopsy is helpful in assessing monoclonality of cells
- Excess of marrow plasma cells
- Kidney biopsy:
- Interstitial infiltrates of abnormal plasma cells
- Proteinaceous casts in distal convoluted tubules (they contain BenceJones proteins, normal plasma proteins, immunoglobulins, Tamm Horsfall protein etc)
- Casts are often surrounded by multinucleate giant cells
- Cells lining the cast containing tubules become necrotic
- S. Protein electrophoresis and paraprotein quantitation
- Prominent M spike (M component) in the region of gamma globulin
- "M"- Earlier stood for "Malignant/ Myeloma", now it stands for "monoclonal"
- It can be whole immunoglobulin (with heavy and light chain) or only immunoglobulin light chain
- It is the most common screening test
- Done on support medium such as cellulose acetate. After passing current, proteins get separated based on their charge.
- Stained with Ponceaus blue and proteins are measured with densitometry.
- Diseases associated with M protein
- Non/ Slowly progressive: Essential monoclonal gammopathy, chronic cold agglutinin syndrome, transient(after inflammation/ BMT), immunodeficiency (particularly T cell)
- Malignant/ Progressive- Plasma cell myeloma, solitary plasmacytoma, light chain amyloidosis, Waldenstrom macroglobulinemia, heavy chain disease, CLL and related lymphomas
- S. Immunofixation electrophoresis
- It is required to determine the immunoglobulin class
- Other indications- Narrow band in beta or gamma region
- Done on agarose gel with impregnated antiserum to specific heavy or light chains (anti alfa, beta, gamma, kappa and lambda) is used.
- Only immunoprecipitates remain on the gel and non precipitated proteins wash out
- Plasma cell dyscrasias secreting only light chains are usually not detectable as limit of immunofixation sensitivity is more than 10 x Normal FLC levels
- S. Free Light Chain Assay
- Measure low levels of FLC in serum
- Measurement is done by nephalometry (based on observation that antigen-antibody complexes form cloudy precipitates). Precipitates can be detected photoelectrically. Varying dilutions of serum are incubated with antibodies specific for anyone of the different immunoglobulin heavy and light chains. After precipitate forms the amount of precipitate is determined by comparison with standard curves produced by precipitating immunoglobulins of known concentration.
- Done when there is strong suspicion of myeloma, but in whom SPE is negative
- It is useful in monitoring as well.
- Abnormal kappa/lambda SFLC ratio is used as surrogate marker for the secretion of monoclonal free light chain
- Abnormal ratio can be observed in SLE and HIV infection and during immune reconstitution following stem cell transplantation
- Normal values (This can differ in different instruments)
- Kappa- 3.3-19.4 mg/L
- Lambda- 5.71-26.3 mg/L
- Kappa:Lambda ratio- 0.26-1.65
- Patients with FLC ratio >100, have high risk of progression to end stage organ damage
- S. Immunoglobulin levels
- Levels of uninvolved immunoglobulin is reduced
- Electrophoresis, Immunofixation electrophoresis and FLC assay can be done on urine and CSF. Test on CSF helps in diagnosis of plasmacytoma of CNS secreting paraprotein.
- Urine microscopic examination: Large, waxy, laminated casts in case of cast nephropathy
- Urine-Bence Jones proteins.
- They are free monoclonal light chains
- They can be detected by
- Heat precipitation – Urine flocculates when heated slowly to 50-60oC and flocculated proteins dissolve on boiling
- Electrophoresis of concentrated urine specimen on cellulose acetate – BJ proteins migrated to globulin region.
- Other conditions in which Bence Jones proteinuria is seen
- Macroglobulinemia
- Amyloidosis
- Lymphoma
- Leukemia
- Immunophenotyping
- Gating: Low side scatter with dim/negative CD45
- Abnormal phenotype and/or monoclonality confirms the diagnosis
- Express monotypic cytoplasmic immunoglobulin- Kappa or Lambda
- Lack surface immunoglobulin
- Markers positive on both normal plasma cells and myeloma cells: CD138, CD38, CD79a, CD40, CD44, CD54, VLA4, VLA5
- Positive on myeloma cells only: CD56, CD58, RHAMM (Hyaluronan mediated motility receptor )
- Positive only on normal cells: CD19, CD45 (Positive on mature myeloma cells), CD11a
- Negative on both normal and plasma cells: CD11b, LFA1, CD20
- Cytogenetics/ FISH:
- Cytogeneticsis difficult due to low proliferative fraction. But FISH can be done.
- Most common abnormality is partial deletion of 13q14 or monosomy 13
- Other abnormalities include:
- Hyperdiploidy with gains in chromosomes – 3, 6, 7, 9, 11, 15 & 19
- Losses from chromosomes - 8, 13, 14, X
- Deletions from chromosomes 6q, 16q, 17p
- Translocations involving IGH gene located on chromosome 14q32 is seen in 60% of patients. These translocations are associated with poor prognosis.
- Molecular studies:
- Antigen receptor genes: Clonal rearrangement of immunoglobulin genes. Single monoclonal rearranged Ig band is the rule in myeloma. High frequency of Ig VH gene somatic mutations is noted. This test is done using PCR.
- Transposition of Cyclin D1 gene into on IgH gamma switch region
- Over expression of Cyclin – D1
- Altered expression of PAX-5 gene on chromosome 9 (This results in loss of CD19).
- Deletion of 17p13 which results in loss of p53
- Activation of NRAS or KRAS - Seen in 40% of cases
- Other mutations seen- myc, FGFR-3, p18, RB1, CDKN2A, PTEN
- Epigenetic changes manifested by DNA methylation are associated with tumor progression
- Deletion of long arm of chromosome 7 (This confers multidrug resistance)
- Quantitative PCR using allele specific oligonucleotides if the gold standard for MRD detection. But this is not practical as laboratory has to synthesize probe specifically for each patient.
- Gene expression profile:
- Helps in
- Better understanding of pathobiology
- Developing new prognostic models
- Identifying new targets for drug development
- Developing personalized medicine approaches
- Technique:
- RNA extracted from CD138+ myeloma cells
- This is hybridized on high density arrays to evaluate expression of genes from the whole transcribed genome
- There are about 10 subclasses of myeloma based on this technique
- Helps in
- X-ray –
- 2 types of lesions
- Diffuse decalcification
- Localized area of bone destruction – Multiple, rounded, discrete, punched out area with no sclerosis at the margin (most commonly seen in skull)
- Skeletal survey must include:
- Chest (PA view)
- AP and lateral view of cervical spine, thoracic and lumbar spine
- AP and lateral - Humeri and femora
- AP and lateral skull
- AP of pelvis
- Order of skeletal involvement: Vertebra (66%), ribs (44%), Skull (41%), Pelvis (28%), Femur (24%), Clavicle (21%)
- Less sensitive compared to cross sectional imaging methods (Whole body CT, MRI, or PET/CT)
- 2 types of lesions
- Whole body low dose CT
- CT has improved sensitivity over X ray, as it picks up lytic lesions measuring <5mm, which are missed on X ray.
- It can be done, even if patient cannot sit/ stand.
- Helps in identifying soft tissue lesions and guides for taking biopsies and doing surgical/ radiotherapy interventions.
- Whole body PET/CT
- To be done if low dose CT is not available.
- More sensitive in picking up extramedullary lesions
- MRI
- It can directly visualise the disease within the bone marrow, rather than its secondary effects on the cortical bone.
- It is technique of choice in case of spinal cord compression
- Avoid Gadolinium if GFR <30mL/min
- Fat pad staining for amyloid
- S. Viscosity- If hyperviscosity is suspected
- HLA typing- If Allo HSCT is planned
- DEXA scan – For measurement of bone density
- Serum calcium level- Elevated
- Serum uric acid level- Elevated
- Serum albumin – Decreased
- Serum CRP- Raised
- Serum beta 2 microglobulin – Raised (reflects tumor burden and high value is associated with poor prognosis)
- Serum Urea, Creatinine – elevated in case of renal dysfunction
- Serum LDH level – Raised
- Markers for bone destruction – Elevated levels of
- TRACP – 5b
- Collagen degradation products – NTX, 1CTP, CTX
- Markers of bone formation – Decreased levels of bone alkaline phosphatase and osteocalcin
Criteria for Diagnosis:
- Clonal bone marrow plasma cells ≥ 10% or biopsy proven bony or extramedullaryplasmacytoma (Clonality by flow/IHC)
AND
- Any one or more of of following myeloma defining events(CRAB):
- Calcium >11mg/dL
- Renal dysfunction: Creatinine- >2mg/dL or creatinine clearance <40ml/min
- Anemia- Hemoglobin <10gm/dL
- Bone lesions: One or more osteolytic bone lesions on skeletal survey/ CT/PET-CT
OR
- Clonal BM plasma cells >60%
OR
- Abnormal FLC ratio >100 (involved kappa) or <0.01 (involved lambda)
OR
- >1 focal lesion on MRI studies (>5mm in size).
Staging:
Myeloma Staging System (Durie –Salmon) | Median Survival |
Stage I :
|
> 60 months |
Stage III : Any one or more of the following
| 23 months |
Stage II : Overall values between I and III | 41 months |
Subclassification : Based on renal function A = serum creatinine< 2 mg/dl B = serum creatinine> 2 mg/dl |
|
International Staging System (ISS) stage:
Stage I-
- Serum beta 2 microglobulin- <3500ng/mL
- Serum albumin >3.5g/dL
Stage II
- Not ISS I or III
Stage III
- Serum beta 2 microglobulin >5500ng/mL
Revised ISS
Stage I- (All)
- Serum beta 2 microglobulin- <3500ng/mL
- Serum albumin >3.5g/dL
- Standard risk cytogenetics by FISH
- Serum LDH- Normal
Stage II
- Not ISS I or III
Stage III (Any one)
- Serum beta 2 microglobulin >5500ng/mL
- High risk chromosomal abnormalities by FISH- t(4:14), t (14:16), del 17p, 1q gains, 1p del
- Raised LDH levels
Prognosis:
- Multiple myeloma is not considered a curable malignancy with current approaches
- Median survival with present treatment- 7 years
- Median survival in untreated symptomatic patients- 6 months
- Poor prognostic markers
- Advanced age with poor PS and presence of co-morbidities
- Raised beta-2 microglobulin levels (which indicates high tumor burden)
- Hemoglobin – Less than 7 gm/dl
- Severe hypoalbuminemia
- High LDH
- Extramedullary disease
- Intractable renal failure
- Thrombocytopenia
- >50% plasma cells in bone marrow
- Plasmablastic morphology
- Circulating plasma cells
- High Ki 67 positivity
- t(4:14), t (14:16), del 17p, 1q gains, 1p del, 13q del
- Some gene expression profiles
- High serum levels of IL6, CRP, hepatocyte growth factor, C terminal cross linked telopeptide of collagen I, TGFbetaand Syndecan
- Tumor resistance
Risk stratification:
- High risk: Any one of the following
- t(4;14), t(14;16), t(14;20), del17p13, or gain 1q
- LDH- >2 x ULN
- Features of plasma cell leukemia (>5% circulating plasma cells)
- Standard risk: Rest all patients
Differential Diagnosis: For increased number of plasma cells in bone marrow
- Aplastic anemia
- Rheumatoid arthritis
- Cirrhosis of liver
- Sarcoidosis
- Secondary carcinoma
- SLE
- Chronic inflammation.
- Primary amyloidosis- Generally havenephrotic syndrome, heart failure, hepatomegaly etc which are not found in multiple myeloma. They have usually less than 20% plasma cells in BM, no lytic lesions on imaging and BJ proteins in urine)
Pretreatment Work-up:
- History
- Examination
- WHO P. S.
- BSA
- BMA and Bx
- IHC/Flow cytometry
- Stage
- Hemoglobin
- TLC, DLC
- Platelet count
- Peripheral smear (% of plasma cells)
- LFT: Bili- T/D SGPT: SGOT: Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid:
- LDH
- β2 microglobulin
- Quantitative Ig: IgG: IgM: IgA:
- SPEP
- S. Immunofixation Electrophoresis
- S. Free Light Chain A
- 24hr Urinary Protein
- Urine- IFEP/PEP
- Skeletal survey/ Whole body Low dose CT/ WB MRI/ WB PET-CT
- BM/Fat for Amyloid
- HIV:
- HBsAg:
- HCV:
- 2D Echo
- UPT
- Cytogenetics
- Plasma cell FISH
- del 13:
- del 17p13:
- t (4;14):
- t (11;14):
- t (14;16):
- 1q21 amplification (>3 copies):
- 1p deletion:
- Dental Examination (Prior to bisphosphonates)
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
Treatment Plan:
Goals of treatment:
- Control of disease
- Maximize quality of life
- Prolong survival
Affordable patient with high risk disease: Daratumumab-bortezomib-lenalidomide-dexamethasone (DVRd) is the preferred regimen(3-6 cycles), followed by early tandem ASCT/Allo SCT in select cases, followed by dual drug maintenance using Lenalidomide and bortezomib.
Extremely frail patient unable to tolerate 3 drugs: 2 drugs (Lenalidomide with Dexa) may be given given, followed by Lenalidomide maintenance.
For all others:
- First line regimens:
- BorLenDex
- CyBorD
- BorThalDex
- DaraLenDex
- CarfilLenDex
- Second Line regimens (Avoid Bortezomib or Lenalidomide if used previously):
- Carfilzomib+Dexa +/-Len
- Dara+Len+Dex
- Carfil+Pom+Dex
- Daratumomab+Bort+Dexa
- Dara+Pom+Dex
- Dara+Carfil+Dex
- Elotuzumab+Len+Dexa
- Ixazomib+Len+Dexa
- Ixazomib+Pom+Dexa
- Isatuximab-irfc/Pomalidomide/dexamethasone
- Benda+Borte+Dexa
- Pomalidomide+Dexa+Bor/Cyclo
- Vincristine+Doxorubicin+Dexamethasone
- Melphalan+Pred+Thal
- Third line regimens:
- Selinexor/bortezomib/dexamethasone
- Bortezomib/liposomal doxorubicin/dexamethasone
- Selinexor/daratumumab/dexamethasone
- Dexamethasone/cyclophosphamide/etoposide/cisplatin
- Dexamethasone/thalidomide/cisplatin/doxorubicin/cyclophosphamide/ etoposide (DT-PACE) ± bortezomib (VTD-PACE)
- Bendamustine/bortezomib/dexamethasone
- Bendamustine/Lenalidomide/dexamethasone
- Bendamustine/carfilzomib/dexamethasone
- Isatuximab-irfc/carfilzomib/dexamethasone
- Fourth line agents:
- Idecabtagenevicleucel
- Ciltacabtageneautoleucel
- Teclistamab-cqyv
- Belantamabmafodotin-blmf
If CNS is involved- RT to localized disease, IT chemo, along with systemic therapy
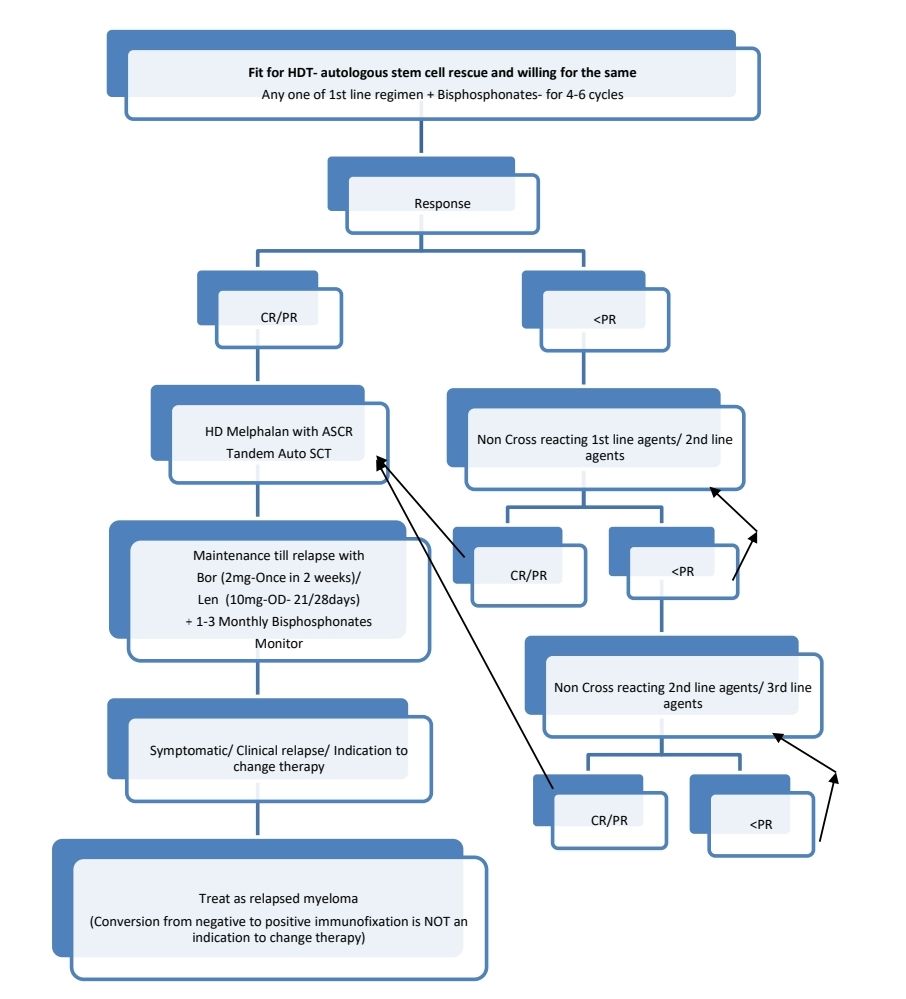
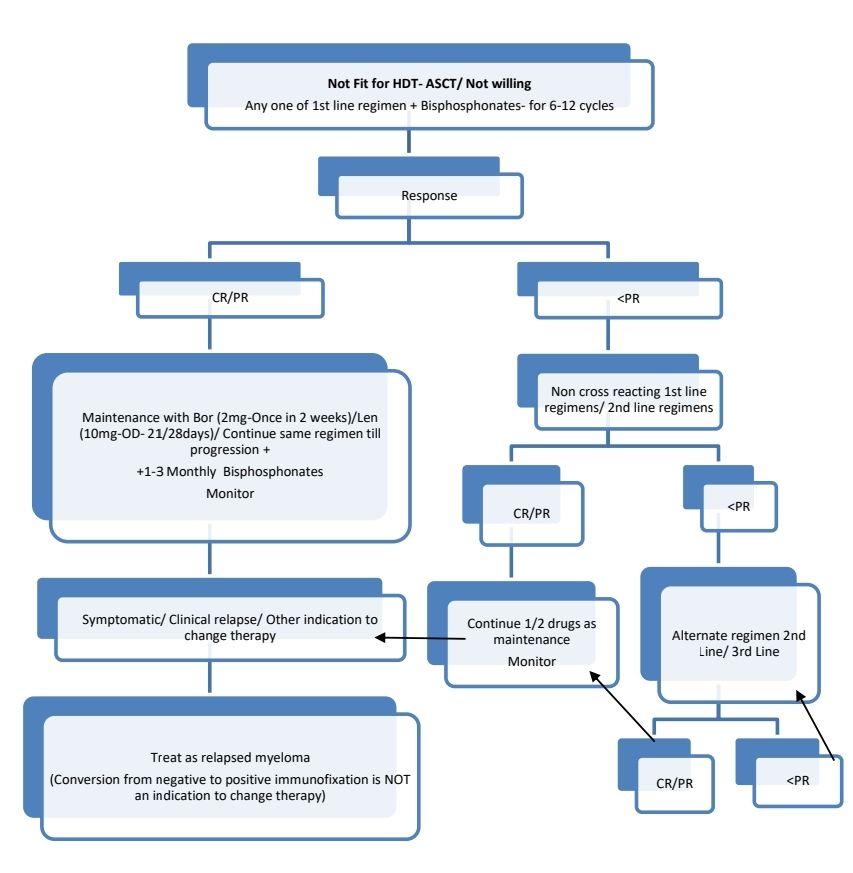
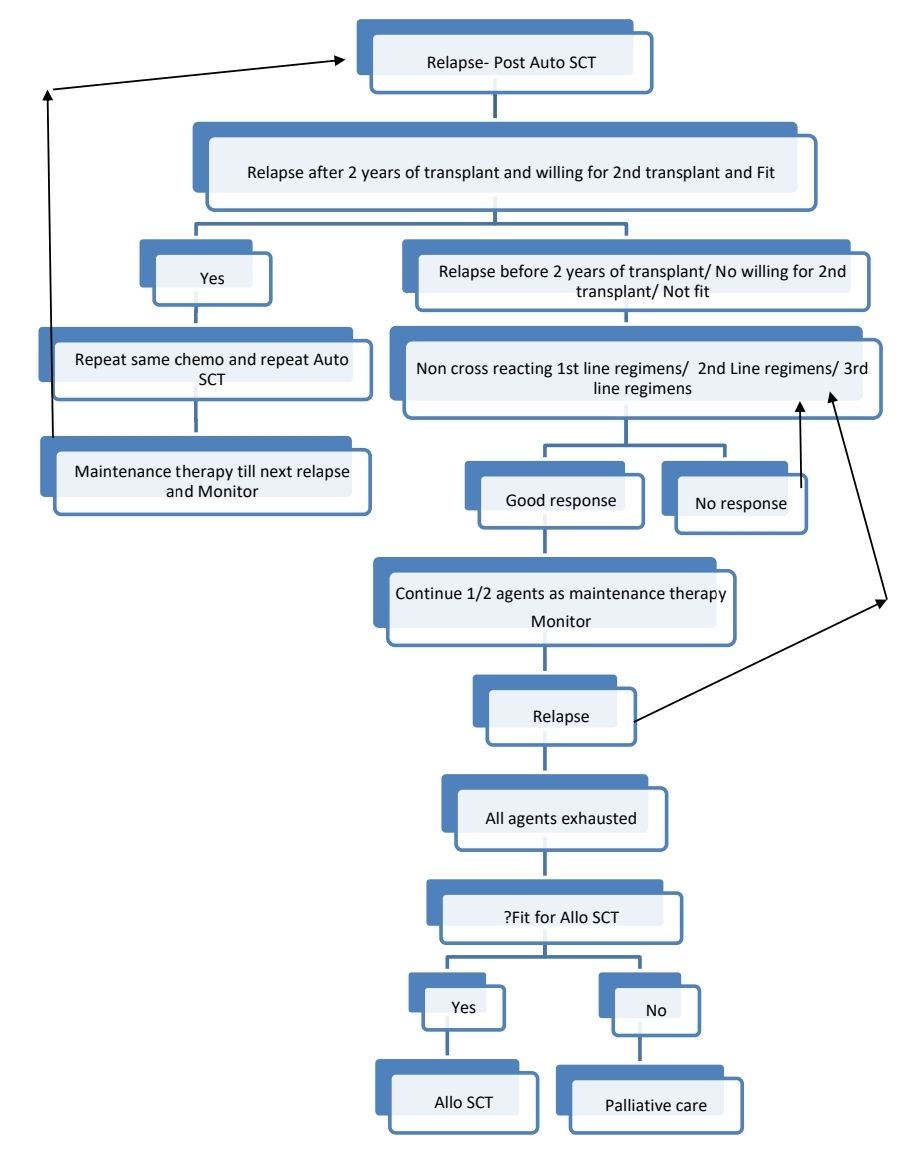
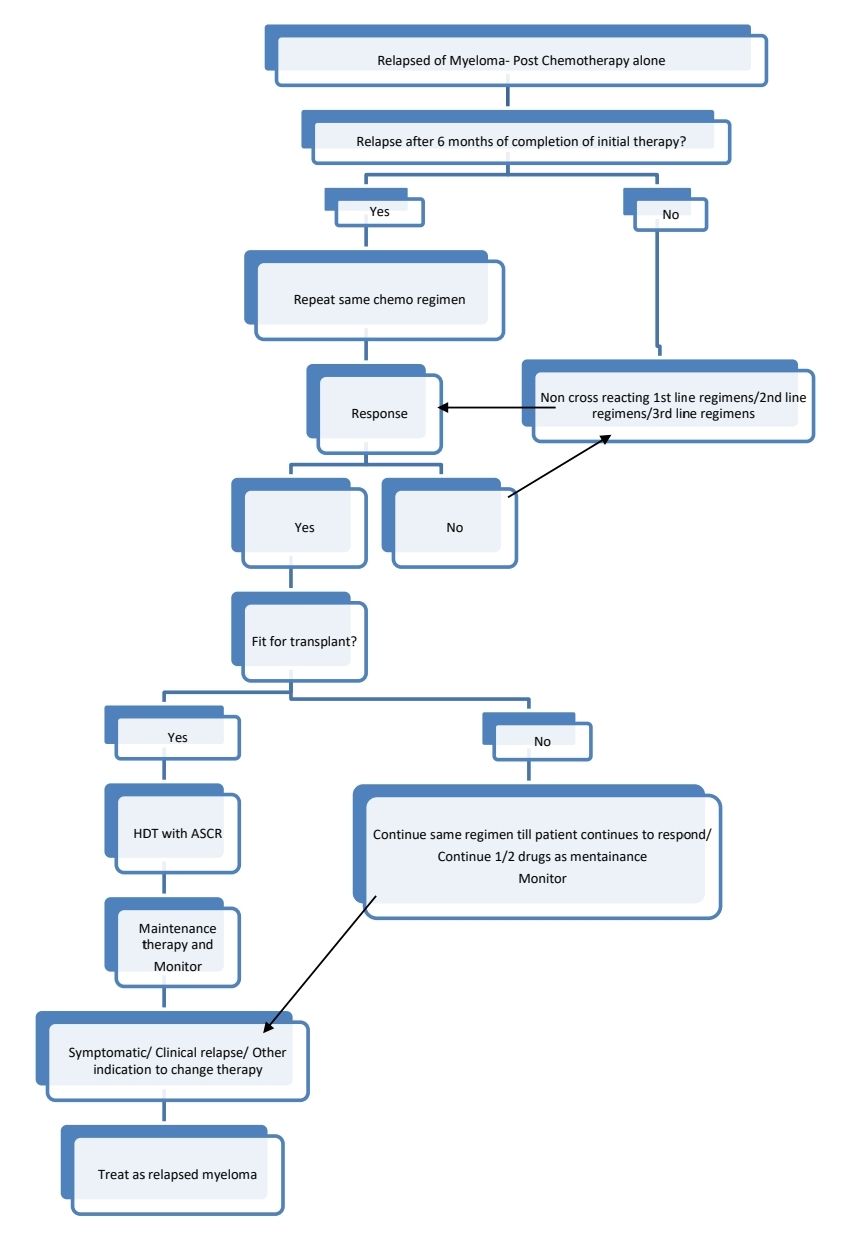
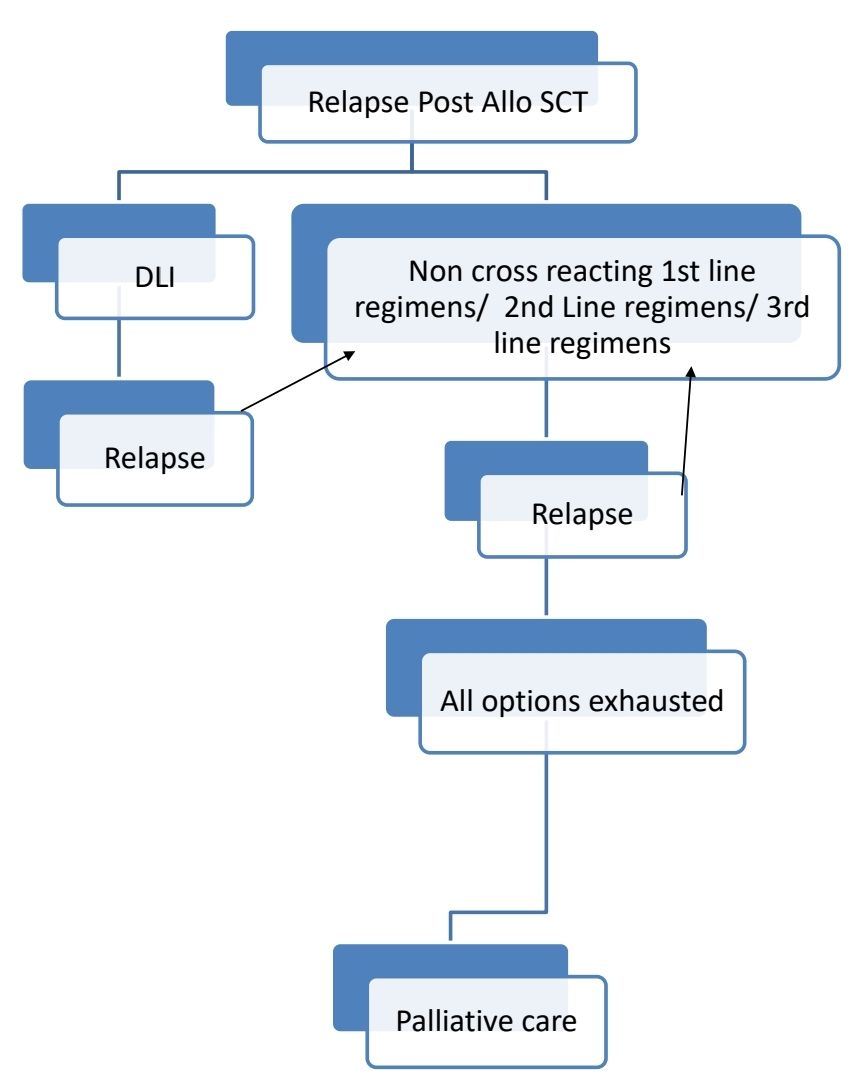
Response Criteria:
- Complete remission (CR)
- Negative immunofixation of serum and urine
- Disappearance of any soft tissue plasmacytomas
- <5% plasma cells in BMA
- Stringent CR
- CR with normal FLC ratio
- Partial response:
- ≥ 50% reduction in Serum M protein or ≥ 50% decrease in difference between involved and uninvolved FLC levels
- ≥ 50% reduction in number of plasma cells in BM (Provided baseline plasma cell percentage is >30%)
Monitoring when on maintenance therapy/otherwise:
- Once a month: History, examination, CBC, Creat, Calcium
- Once in 3 months: SPE
- If clinically indicate: BMA and biopsy imaging, Immunofixation electrophoresis, SFLC Assay
Progressive disease is identified by rise in monoclonal protein/ worsening SFLC ratio. This does not require immediate change of treatment.
Therapy for relapsed disease is indicated if:
- Clinical relapse (Development of CRAB features)
- Extramedullary disease
- Rapid rise of paraproteins: Doubling of M Protein over 2-3 months with an increase in the absolute levels of M protein of >1gm/dL, confirmed by 2 consecutive tests.
- Absence of myeloma-defining events or amyloidosis
- Bone marrow plasma cells- >20%
- M Protein- >2gm/dL
- SFLC ratio- >20
- Absence of myeloma-defining events or amyloidosis
About Each Modality of Treatment:
Chemotherapy Regimens for treatment of myeloma
- Triplet regimens are usually used as standard therapy, however elderly/ frail patients may be treated with doublet therapy
- Currently used agents include
- Alkylators- Melphalan, cyclophosphamide, Bendamustine
- Corticosteroids- Dexamethasone, prednisolone
- Anthracyclins- Doxorubicin
- Immunomodulatory drugs- Thalidomide, lenalidomide, pomalidomide
- Proteasome inhibitors- Bortezomib, Carfilzomib, Ixazomib, Oprozomib
- Monoclonal antibodies- Daratumomab (Anti-CD38), Elotuzumab (Anti CS1 Glycoprotein), Indatuximab (Anti CD138)
- Usually 4-6 cycles are given prior to reassessment for response
- In case of pre-existing severe neuropathy- Avoid thalidomide, bortezomib and Vincristine.
- CyBorD
- Frequency: 28 days
- Inj. Dexamethasone 40mg in 100ml NS over 1hr- on day 1, day 8, day 15 and day 22
- Inj. Bortezomib- 1.3mg/m2- in running saline- on day 1, day 8, day 15 and day 22
- Inj. Cyclophosphamide- 500mg- in 500ml D5% over 2hrs- on day 1, day 8, day 15 and day 22
- Inj. Zolendronic acid- 4mg- in 100ml NS over 30min- on day 1
- Dose adjustments:
- ANC- <1000/cmm or Platelet count- <50,000/cmm- Hold chemo until recovery and restart at dose- Cyclophosphamide 400mg/300mg and Bortezomib- 1mg/m2
- Creatinine- >3.4mg/dL despite of vigorous hydration- Omit cyclophosphamide
- Creatinine clearance- <30ml/min- Bortezomib- Give 50% of dose
- Peripheral neuropathy
- Grade 2- Bortezomib- Give 1mg/m2
- Grade 3- Stop bortezomib till symptoms subside and then restart at 0.7mg/m2
- Grade 4- Discontinue bortezomib
- Severe diarrhea- Delay until diarrhea resolves. Restart bortezomib at 1gm/m2
- LenBorD
- Frequency: 21 days
- Inj. Dexamethasone 40mg in 100ml NS over 1hr- From Day 1 to Day 4. (4 days)
- Inj. Bortezomib- 1.3mg/m2- in running saline- on day 1, day 4, day 8 and day 11
- Cap. Lenalidomide- 25mg- PO- OD- From day 1 to day 14. (14 days)
- Inj. Zolendronic acid- 4mg- in 100ml NS over 30min- on day 1
- Dose adjustments:
|
| Dexamethasone | Bortezomib | Lenalidomide | Zolendronic acid |
ANC (/cmm) | <500 | -- | Give 1mg/m2 | Stop till recovery and restart at 10mg/day |
|
Platelet count (/cmm) | <30,000 |
|
| Stop till recovery and restart at 10mg/day |
|
Creatinine clerance (ml/min) | 30-50 |
|
| Give 10mg/day | Give 3mg |
| <30 |
| Give 50% of dose | Give 5mg/day | Omit |
Peripheral neuropathy | Grade 2 |
| Give 1mg/m2 |
|
|
| Grade 3 |
| Stop till recovery and restart at 0.7mg/m2 |
|
|
| Grade 4 |
| Omit |
|
|
Severe diarrhea |
|
| Stop till diarrhea subsides and ten start at 1mg/m2 |
|
|
- MPT
- Frequency: 28 days
- Tab. Melphalan- 7mg/m2- OD- From Day 1 to Day 4 (4 days)
- Tab. Prednisolone- 50mg- OD- From Day 1 to Day 4 (4 days)
- Tab. Thalidomide 50mg HS, then gradually increase to 200mg HS- From Day 1 to Day 28
- Tab. Aspirin- 75mg- OD- From Day 1 to Day 28
- Inj. Zolendronic acid- 4mg- in 100ml NS over 30min- on day 1
- Dose adjustments:
|
| Melphalan | Prednisolone | Thalidomide | Zolendronic acid |
ANC (/cmm) and / or Platelet count (/cmm) (Monitor every week) Hold chemo till ANC >1300 and Platelet count >75,000 and decrease the dose in subequent cycles. If no improvement till 6 weeks- change to alternate chemotherapy. | 500-1000/ 25,000-75,000 | Give for 3 days |
|
|
|
| <25,000/ <500 | Give for 2 days |
|
|
|
Creatinineclerance (ml/min) | 30-50 | Give 50% of dose |
|
| Give 3mg |
| <30 | Avoid |
|
| Omit |
Peripheral neuropathy | Grade 2 or more |
|
| Stop |
|
- Carfil-Len-Dex
- Frequency: 28 days
- Inj. Dexamethasone- 40mg in 100ml NS over 30min- On Day 1, Day, 8, Day 15 and Day 22.
- Inj. Carfilzomib- 27mg/m2 in 100ml D5% over 10min (start within 30min of Dexamethasone)-
- For Cycles 1- 12- On Day 1, Day 2, Day 8, Day 9, Day 15 and Day 16.
- For Cycles 13- 18- On Day 1, Day 2, Day 15 and Day 16.
- After 18 cycles- discontinue Carfolzomib and continue Len Dexuntil disease progression/ acceptable toxicity.
- Tab. Lenalidomide- 25mg- OD- From day 1 to Day 21 (21 days)
- Tab. Aspirin- 75mg- OD- From day 1 to Day 21 (21 days)
- Inj. Zolendronic acid- 4mg- in 100ml NS over 30min- on day 1
- Dose adjustments:
Cap BSA at 2..2m2
|
| Dexamethasone | Carfilzomib | Lenalidomide | Zolendronic acid |
Elderly |
| Give 20mg |
|
|
|
ANC (/cmm) or Platelet count (/cmm) Delay until ANC >1000 and Platelet count >30,000 | <1000/ <30,000 |
| First time- Restart in same dose Recurrence- Restart at 20mg/m2 Again recurrs- 15mg/m2 Again recurs- Discontinue | First time- Restart at 20mg/day Same counts on day 15- Omit for rest of cycle and next cycle give 15mg/day Recurs again on day 15- Omit for rest of cycle and next cycle give 10mg/day Recurs again on day 15- Omit for rest of cycle and next cycle give 5mg/day Recurs again on day 15- Omit for rest of cycle and next cycle give 2.5mg/day |
|
Creatinineclerance (ml/min) | 30-60 |
|
| 10mg/day | Give 3mg |
| 15-30 but no HD |
|
| 15mg/day | Omit |
| 15-30 and requires HD |
|
| 5mg/day- after HD | Omit |
| <15 |
| Hold till >15 | 5mg/day | Omit |
- Pomalidomide- Cyclo-Dex
- Frequency: 28 days
- Inj. Zolendronic acid- 4mg- in 100ml NS over 30min- on day 1
- Tab. Pomalidomide- Start with 2mg- OD and gradually increase to 4mg OD- From days 1 to 21
- Tab. Dexamethasone- 40mg- On days 1, 8, 15 and 22.
- Tab. Cyclophosphamide- 400mg- on days 1, 8, and 15 (Premedicate Tab. Emeset- 8mg)
- Tab. Aspirin- 75mg- OD
- Dose adjustments:
- ANC- <1000/cmm or Platelet count- <50,000/cmm- Hold chemo until recovery and restart at dose- Cyclophosphamide 300mg and Pomalidomide at 3mg. If this repeats hold until recovery and restart at dose- Cyclophosphamide 300mg and Pomalidomide at 2mg. If this repeats hold until recovery and restart at dose- Cyclophosphamide 300mg and Pomalidomide at 1mg. If this repeats discontinue pomalidomide.
- Creatinine- >3.4mg/dL despite of vigorous hydration- Omit cyclophosphamide. Pomalidomide has no renal dose adjustment.
- Severe rash- Discontinue pomalidomide
- VAD
- Frequency: 21 days
- Inj. Vincristine (0.4mg/m2) + Inj. Doxorubicin (9mg/m2)- in 500ml NS over 24 hrs- From Day 1 to Day 4 (Continuous infusion for 4 days)
- Tab. Dexamethasone- 40mg- OD from Day 1 to Day 4 and then from Day 12 to Day 15.
- Inj. Zolendronic acid- 4mg- in 100ml NS over 30min- on day 1
- Dose adjustments:
- ANC- <1000/cmm or Platelet count <50,000/cmm- Hold next cycle until ANC- >1000/cmm and Platelet count >50,000/cmm (Unless cytopenia is thought to be due to myeloma per se)
- Significant neuropathy- Omit vincristine
- Bilirubin- >3gm/dL- Consider decreasing the dose of vincristine and doxorubicin.
- Severe steroid related side effects- Omit dexamethasone from day 12 to day 15.
- Creatinine clearance (ml/min)
- 30-50- Zolendronic acid- Give 3mg
- <30- Omit Zolendronic acid
- Dara-Len-Dex
- Frequency: 28 days
- Premedication- Tab. Montelukast- 10mg- Stat,Tab. Paracetamol- 1gm-Stat, Tab. Avil- Stat
- Tab. Dexamethasone 20mg
- Cycle- 1 and 2- Days- 1&2, 8&9, 15&16, 22&23
- Cycle- 3 to 6- Days- 1&2, 8&9, 15&16, 22&23
- Cycle 7 onwards- Days 1 and 2, 40mg on days 8, 15, and 22.
- Inj. Daratumumab- 1800mg- SC- over abdomen- over 3-5min
- Cycle- 1 and 2- Days- 1, 8, 15, 22
- Cycle- 3 to 6- Days- 1 and 15
- Cycle 7 onwards- Day 1
- Cap. Lenalidomide- 25mg- PO- OD- From day 1 to day 21
- Inj. Zolendronic acid- 4mg- in 100ml NS over 30min- on day 1
- Dose adjustments:
|
| Dexamethasone | Dara | Lenalidomide | Zolendronic acid |
ANC (/cmm) | <1000 | -- | Stop till recovery and restart at 10mg/day |
| |
Platelet count (/cmm) | <30,000 |
|
| Stop till recovery and restart at 10mg/day |
|
Creatinine clerance (ml/min) | 30-50 |
|
| Give 10mg/day | Give 3mg |
| <30 |
| Give 5mg/day | Omit |
Autologous Stem Cell Transplantation
- It is associated with long treatment free survival and excellent quality of life.
- Renal dysfunction or advanced age are not contraindications for auto SCT
- Compared to chemotherapy alone, ASCT has superior progression free survival (median >20 months) but same overall survival.
- Should be done if patient is in CR or PR
- 2 types:
- Early HCT: Done as a part of initial therapy
- Late HCT: Delayed until first relapse
- PBSCs are harvested after mobilization with growth factors. Sometimes cyclophosphamide (1.5-4gm/m2- especially if lenalidomide is used in induction) and plerixafor are used.
- Generally sufficient stem cells to support 2 ASCTs are collected. Second ASCT is to be done in case of relapse.
- After high dose melphalan (usually 200mg/m2, needs renal dose adjustment), stem cell infusion is done
- High response rate – CR up to 30%, 7% molecular remission is seen even in patients who are refractory to conventional treatment
- Event free survival- 13 months
- Median survival – 50 months
- TRM < 2%
- Tandem transplant- Second course of high dose chemotherapy and autologous stem cell transplant, done within 6 months of first course. There is no clear data on benefit of this on improving overall survival.
Allogeneic SCT
- Myeloablative conditioning- only for age <40years who have achieved atleast PR with initial therapy.
- Non myeloablative/ RIC regimens may be used for elderly patients.
- Too risky but has advantage of Graft Vs Myeloma effect. Hence long term disease free survival
- Median survival – 50 months
- TRM – 30 – 50%
- Chances of CR- 50%
- Limited role as more than 75% patients of myeloma are aged >55years and have multiple comorbidities.
- It is the only treatment option with potential of cure
- DLI can be used for relapse or for mixed chimerism
- MUD/Haplo SCT is not recommended, except in the context of a clinical trial.
Maintenance therapy
- Prolongs remission duration, thereby life expectancy
- Lenalidomide (10mg- 21/28days)/ Fortnightly Bortezomib (2mg)/ Thalidomide (100mg- Daily) are used.
- Must be combined with bisphosphonates once a month
Supportive Care:
- Bone disease:
- Bisphosphonates- Given to all patients and continued indefinitely even after treatment except those who are in CR following transplant.
- Due to concerns of osteonecrosis of jaw, do dental examination prior to starting.
- Calcium and vitamin D supplementation also should be given.
- Stabilization and subsequent radiotherapy for fractures (8Gy single fraction- Improves pain control and promotes healing of fracture site)
- Pain:
- 67% of myeloma patients have pain at diagnosis.
- Refer to supportive care section
- Hypercalcemia: Refer to supportive care section
- Tumor lysis prophylaxis and treatment: Refer to supportive care section
- Renal impairment:
- Rehydration- To achieve urine flow of 3lit/day
- Correct hypercalcemia
- Discontinue nephrotoxic drugs like NSAIDS
- Start antimyeloma therapy immediately (High dose dexamethasone to be started while decision about chemotherapy is being made)
- Dialysis
- New dialysis filters are available that can remove light chains.
- Nephropathy is reversible in 50% of patients.
- For renal transplant myeloma is not a contraindication
- Radiotherapy –
- Indications:
- Extramedullaryplasmacytoma
- Painful destruction of vertebral body/ pathological fracture
- Spinal cord compression
- Doses:
- Low dose- RT- 8Gy x 1 fraction
- 10-30Gy in 2-3Gy fractions
- Indications:
- Surgery: Indications:
- Spinal cord compression
- Unstable vertebral fractures
- Anemia:
- Transfusion support
- Erythropoietin- 5000 U- Twice a week – SC or Darbepoetin 6.25 microgm/kg every 3 weeks
- Decreases transfusion requirement
- Given to maintain Hemoglobin around 11gm/dL. With higher hemoglobin, risk of thrombosis increases.
- Hyperviscosity – Plasma exchange
- Infections
- Antibiotics
- Prophylactic IV- immunoglobulins if patient has recurring infections- 500mg/kg every month, for up to 6 months.
- Pneumococcal and influenza vaccines
- PCP, herpes and antifungal prophylaxis
- Coagulation
- Prophylactic anticoagulation for patients receiving thalidomide/ lenalidomide
- Aspirin- 75mg- OD if patient is receiving thalidomide/ lenalidomide
- If additional risk factors forthrombosis(personal/family history of DVT, Obesity, co-morbidities, recent surgery) are present, avoid Thal/Len or give LMWH/Warfarin (target INR-2-3)/ Rivaroxaban-10mg-OD as thromboprophylaxis. It should be continued till disease is under control (generally for 4-6 months).
- Avoid anticoagulation/ antiplatelet agents, if there is risk of bleeding.
- Prophylactic anticoagulation for patients receiving thalidomide/ lenalidomide
- Cord compression: Refer supportive care section
- Peripheral neuropathy: Neuromodulatory drugs may be used
- Gabapentin- 800mg/day. It suppresses BM, hence must be used with caution if AutoSCT is planned
- Pregabalin
- Oxcarbamazepine
IgD Myeloma
- 1.8% of all myelomas
- Very small or no M band
- BJ Proteins, extramedullary involvement, lytic lesions and amyloidosis are common
IgM Myeloma
- 0.4% of all myelomas
- Must be differentiated from Waldenstrom'smacroglobulinemia
- High incidence of t(11:14)
- Poor prognosis
Smouldering myeloma
- Fulfills the criteria for multiple myeloma but no myeloma defining (CRAB) events/ amyloidosis
- It progresses to multiple myeloma at a rate of approximately 10% per year.
- Criteria for diagnosis: Essential:
- Serum monocional protein (IgG or IgA) ≥ 3 g/dL, or urinary monoclonal protein ≥ 500 mg per 24 h and/or clonal bone marrow plasma cells 10-60%
AND
- High risk: If 2 or more of following features are present-
- Management
- Observe only- 3-6 monthly CBC, Creatinine, Calcium, SPEP, SIFE and SFLC Assay. If clinically indicated do BMA and biopsy and WB MRI or PET-CT.
- If there is progression, treat like multiple myeloma
- For high risk patients: Same as above/ Lenalidmide monotherapy may be considered
Indolent myeloma
- Criteria for diagnosis of multiple myeloma are met, but
- Patients have up to 3 lytic bone lesions, without bone pain
- M component is at intermediate level.
- Normal Hb, serum calcium and creatinine.
- No evidence of infection
Non secretory myeloma (1% cases)
- In 85% of cases, there is synthesis, but no secretion of immunoglobulin
- Presentation is same as multiple myeloma but renal insufficiency is not seen.
- Fulfills all criteria of multiple myeloma (including >10% clonal plasma cells in bone marrow) but no monoclonal immunoglobulin and normal SFLC assay.
Plasma cell leukemia
- Presence of ≥ 5% circulating plasma cells in peripheral blood smears in patients otherwise diagnosed with plasma cell myeloma
- Unlike myeloma, CD56 is negative. Some are cyclin D1-Positive.
- Can be de novo or may appear as late feature in the course of multiple myeloma.
- Higher incidence in Light chain only, IgD or IgE myelomas
- Morphology: Plasma cells may be small with little cytoplasm and may resemble plasmacytoid lymphocytes.
- Presentation- Osteolytic lesions are less frequent, Lymphadenopathy and organomegaly are common.
- Prognosis: Poor
- Treatment: Treat as ver high risk myeloma. Bortezomib containing chemotherapy followed by autologous stem cell transplant
Solitary plasmacytoma
- Localized osseous or extraosseous growth of plasma cells, identical to those seen in plasma cell myeloma without evidence of multiple myeloma or end organ damage due to plasma cell neoplasia
- Account for 3-5% of plasma cell neoplasms
- M:F= 1.87
- Mean age- 60 years
- Types
- Solitary plasmacytoma of bone: Involves vertebrae, ribs, skull, pelvis and femur. There is lytic bone lesion but without bone marrow involvement. Present with bone pain at the site of the lesion and pathological fracture
- Extraosseous plasmacytoma: Involves upper respiratory tract (especially nasopharynx& sinuses), GIT, urinary bladder, CNS, breast, thyroid, testis, parotid, LN & Skin.
- Investigations (Mostly done to rule out multiple myeloma):
- CBC, Electrolytes, Ca, RFT
- SPE: M component is present in 1/3rd of cases
- SIFE and SFLC assay
- Biopsy and IHC:
- Plasma cells are present in sheets
- Markers helpful in establishing monoclonality- CD19, CD56, CD27, CD17, Cyclin D1
- Markers in differentiating plasma cells from B cells: CD138, MUM1/IRF4, CD20, PAX5
- Bone marrow aspiration and biopsy
- Skeletal survey/ MRI spine with pelvis
- Diagnostic criteria- Each of the following must be present
- Biopsy proven plasmacytoma with biopsy proven clonal plasma cells
- Absence of clonal plasma cells in BM aspirate (<10% )
- Absence of end organ damage that can be attributed to underlying plasma cell neoplasm (CRAB features)
- Absence of other lytic lesions on WB-PET-CT or WB- MRI.
Prognostic categories:
Variables | Risk Group | 5 year progression rate to multiple myeloma |
Normal SFLC ratio | Low | 13% |
Either variable abnormal | Intermediate | 26% |
Abnormal SFLC ratio | High | 62% |
- >50% of patients with plasmacytoma may progress to multiple myeloma in 2-4 years. This chance is 30% with extra osseous plasmacytomas.
- Plasmacytoma with minimal BM involvement (<10% clonal plasma cells) have high risk of developing multiple myeloma
- Treatment:
- Radiation – 40 – 50 Gy in 1.8-2Gy/fraction to involved field (with margin of atleast 2cm) with/without surgery
- Surgery is advised if there is structural instability or if there is neurological compromise due to mass effect. This should be done prior to radiation.
- Decompression laminectomy
- Spinal fusion
- Intramedullary rod fixation of long bones
- Plasmacytoma with minimal BM involvement may be treated similar to multiple myeloma
- Local recurrence chance is < 5%
- Follow-up: Every 3-6 monthly with CBC, Creatinine, Calcium, LDH, SPEP, SIFE and SFLC Assay- If clinically indicated BMA and Biopsy- If there is progressive disease treat as multiple myeloma
Multiple Plasmacytomas
- Criteria for diagnosis:
- No M protein in serum/ urine
- >1 localized area of bone destruction/ extramedullary tumor of clonal plasma cells
- Normal bone marrow
- Normal skeletal survey/ MRI spine
- No related organ/ tissue impairment
- Treatment - Not clear. Depends on patient's age, sites involved, number of lesions, disease free survival. Options of treatment are
- Systemic therapy +/- autoSCT
- Radiotherapy alone
Plasma cell neoplasms with associated paraneoplastic syndrome
POEMS Syndrome
(Osteosclerotic Myeloma, Crow – Fukase syndrome)
- It is associated with:
- Polyneuropathy- Sensory motor demyelination
- Organomegaly- Hepatosplenomegaly
- Endocrinopathy- Diabetes, Testicular atrophy
- Monoclonal gammopathy- Usually IgG lambda or IgA lambda
- Skin changes – Hyperpigmentation, hypertrichosis.
- Epidemiology:
- M:F- 1.4:1
- Median age- 50 years
- Etiology:
- Imbalance of proinflammatory cytokines
- VEGF produced by tumor cells induces angiogenesis
- ?HHV 8 infection
- Other clinical features:
- Edema and serous cavity effusions
- Papilledema
- Weight loss
- Fatigue
- Clubbing
- Bone pain
- Arthralgia
- Rarely- Renal insufficiency, thrombocytosis, hypercalcemia, pathological fractures
- Investigations
- Bone marrow biopsy- Osteosclerotic lesion. Plasma cell infiltrate with thickened bone trabeculae and fibrosis
- Lymph node biopsy- Resemble plasma cell variant of Castleman disease . Follicular proliferation with regressed (hyaline vascular) & reactive follicles, and interfollicular plasma cell accumulation
- Immunophenotyping- MonoclonalcytoplasmicIg, light chain is lambda.
- Diagnostic criteria
- Mandatory criteria
- Polyneuropathy (typically demyelinating)
- Monoclonal plasma cell proliferative disorder
- Major criteria (At least 1 required)
- Castleman disease
- Osteosclerotic bone lesions
- VEGF elevation
- Minor criteria (At least1 required)
- Organomegaly
- Endocrinopathy
- Skin changes
- Papilloedema
- Thrombocytosis
- Extravascular volume overload
- Mandatory criteria
- Treatment:
- Chemotherapy similar to myeloma, followed with HDT and AutoSCT
- Bevacizumab- AntiVEGF antibody
TEMPI Syndrome
- Features include:
- Telangiectasia
- Erythrocytosis with high EPO levels
- Monoclonal gammopathy
- Perinephric fluid collections
- Intrapulmonary shunting
- Diagnostic criteria (All 3 major and at least 1 minor must be present)
- Major:
- Telangiectasias
- Elevated erythropoietin and erythrocytosis
- Monoclonal gammopathy (IgG kappa)
- Minor:
- Perinephric fluid
- Intrapulmonary shunting
- Major:
- Often it is associated with thrombosis
AESOP Syndrome
(Adenopathy and extensive skin patch overlying plasmacytoma)
- Skin overlying solitary plasmacytomas involving bone are affected by violaceous patches.
- Lesions are usually seen on thorax
- Associated with polyneuropathy and lymphadenopathy
Monoclonal Gammopathy of undetermined significance (MGUS)
- Asymptomatic
- M component is discovered unexpectedly during serum protein electrophoresis
- No evidence of multiple myeloma/ amyloidosis/ Waldenstrom'smacroglobulinemia/ other related disorders
- No end organ damage
- Prevalence
- 3% of people above 50 years
- 5% of above 70 years
- 10% of above 80 years
- 75% of MGUS paraproteins are IgG
- 25% develop – Plasma cell myeloma/ Primary amyloidosis/ Macroglobulinemia
- 2 types
- Lymphoid/ lymphoplasmacytoid MGUS- Usually IgM
- Plasma cell MGUS- Other than IgM
- IgG- 70%, IgM-15%, IgA- 12%, Biclonal- 3%, Only light chains- 20%
- Criteria for Diagnosis (All must be met)
- Serum M protein of < 3g/dl
- <10% bone marrow infiltration by clonal neoplastic cells
- No evidence of anaemia, constitutional symptoms, hyperviscosity, lymphadenopathy, lytic bone lesions, renal insufficiency, or hepatosplenomegaly that can be attributed to the underlying lymphoproliferative disorder
- If paraproteins are >30gm/L or Marrow plasma cells are >10%, with no CRAB features, it is categorized as smoldering myeloma
- Investigations:
- Evaluate thoroughly to rule out myeloma- CBC, RFT, Ca, SIFE. SFLC assay
- Skeletal survey
- Bone marrow aspiration and biopsy
- Normal mature plasma cells, without nucleoli which constitute<10% of nucleated cells in BM
- Express monotypic cIG of the same isotype as the M component in the serum and urine
- Flow cytoemtry: 2 populations of plasma cells
- Normal (Polyclonal)- CD38+ (bright), CD19+, CD56-
- Abnormal (Monoclonal)- CD19-, CD56+/-, Weak CD38+
- Cytogenetics- Difficult to get metaphases
- Risk of transformation to multiple myeloma is 1% per year
- High risk features:
- M Protein- >1.5gm/dL
- Non IgG MGUS
- Abnormal SFLC ratio
Risk | Number of risk factors | Absolute risk of progression at 20years |
Low | 0 | 5% |
Low-Int | 1 | 21% |
Int-High | 2 | 37% |
High | 3 | 58% |
- No treatment needed (Early intervention with len-dexa is being tried in high risk patients)
- Follow up every 6-12 months: With History, SPE, CBC, RFT and Calcium.
Monoclonal gammopathy of clinical significance
- It is same as MGUS, but is associated with potentially severe organ damage, due to toxicity of the monoclonal immunoglobulin or to other mechanisms
- They include
- Monoclonal gammopathy of renal significance
- Atypical bullouspephigoid with MGUS
- MGUS with corneal crystallopathy
- Paraprotein related neuropathy
- They need treatment to preserve involved organ function
Monoclonal gammopathy of renal significance
- It includes any B-Cell or plasma cell clonal disorder that does not fulfill criteria for cancer yet produces a nephrotoxic monoclonal immunoglobulin that leads to kidney injury.
- In this condition, there is low tumor burden, yet, there is renal injury due to presence of monoclonal immunoglobulins.
- Mechanisms of renal damage include:
- Cast nephropathy (this requires high levels of serum free light chains and often patients with this findings have overt multiple myeloma)
- Amyloid deposition
- Intracytoplasmic crystals of light chains
- C3 Glomerulopathy
- Activation of alternate complement pathway: Proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID)
- Thrombotic microangiopathy
- 40-45% of patients with MGUS have MGRS related diseases if renal biopsy is done. Renal biopsy is indicated in MGUS is there is
- High urinary protein level (>1.5gm/day)
- Abnormal free light chain ratio
- Microscopic hematuria
- AKI-Stage 3
- eGFR <60mL/min
- Subtypes of MGRS
- Always associated with MGRS
- Crystal-storing histiocytosis
- Crystalglobulin-induced nephropathy
- Ig-related amyloidosis
- LC proximal tubulopathy
- Monoclonal Ig deposition disease
- Proliferative glomerulonephritis with monoclonal Ig deposits
- Frequently associated with MGRS
- C3 glomerulopathy with MG
- Cryoglobulinaemic glomerulonephritis (type I and [mostly] type II)
- Monoclonal immunotactoid glomerulonephritis
- Thrombotic microangiopathy with MG
- Rarely associated with MGRS
- Monotypic membranous nephropathy
- Monotypic anti-GBM disease
- Monotypic IgA nephropathy / Henoch Schönlein purpura nephritis
- Always associated with MGRS
- If MGRS is idenfied on renal biopsy, these patients must undergo
- S. Protein electrophoresis
- S. Immunofixation electrophoresis
- S. Free light chain assay
- Bone marrow aspiration and biopsy
- Flow cytometry on BM sample for identification of clonal population
- PET CT to detect focal lesion outside BM
- Criteria for diagnosis:
- Essential
- Kidney biopsy demonstrating injury as a result of a monoclonal immunoglobulin
- Proteinuria > 1 g/d comprised mostly of albuminuria
- Progressive acute or subacute kidney injury
- Desirable
- Lack of lytic bone lesions
- No extramedullary plasmacytoma
- No hypercalcemia secondary to bone lesions
- No anaemia with haemoglobin < 10 g/dl
- Bone marrow plasma cells < 60%
- Involved to uninvolved free light chain ratio < 100
- No hyperviscosity
- No bulky lymphadenopathy
- No thrombocytopenia (< 1lac/cmm)
- Essential
- Treatment (Depends on nature of the clone detected)
- If there is <1gm/day proteinuria and no renal damage: Wait and watch along with ACE inhibitors as anti-proteinuric therapy
- CLL clone/ IgM Deposits: Chemotherapy along with rituximab
- Plasma cell clone/ non-IgM Deposits: Myeloma type of treatment (Bortezomib based)
- If there is ESRD- Renal transplantation is the best option, after eradicating monoclonal gammopathy
- If there is no favourable response after 2-3 cycles of chemotherapy, change the therapy to target the alternate clone.
Heavy chain diseases
- Characterized by the production of a shortened monoclonal immunoglobulin heavy chain, with typically no light chain.
- Heavy chain is often truncated with absence of portions of the variable heavy chain domain
- As variable sized proteins are produced, M spike is is often not seen, but immunofixation can identify abnormal chains.
- 3 types:
- Mu HCD: Extremely rare, resembles CLL/SLL, Some associated with MYD88 p. L265P mutation, presents with splenomegaly, hepatomegaly, lymphadenopathy or lytic bone lesion, BM shows IgM positive small lymphocytes, wait and watch, if causing problems- treat as CLL.
- Gamma HCD (Franklin disease): Rare, resembles lymphoplasmacytic lymphoma/ SMZL, may be associated with autoimmune diseases, presents with fever, weight loss, and generalized lymphadenopathy, IgG is elevated, wait and watch, if causing problems- Rituximab based therapy
- Alpha HCD (Selingmann’s disease, Mediterranean abdominal lymphoma, immuno proliferative small intestinal disease): Caused by infections such as Helicobacter pylori, Vibrio cholera, Campylobacter jejuni, or intestinal parasites, Presents with chronic watery diarrhea, weight loss, malabsorption, abdominal pain, finger clubbing, vomiting and fever, IgA is elevated, duodenum is commonly involved, Treatment includes 6 months course of antibiotics. If no response/ systemic disease/ large cell transformation use R-CHOP.
Idiopathic Capillary Leak Syndrome
Introduction:
- It is a rare disease charectorised by episodes of severe hypotension, hypoalbuminemia and hemoconcentration
- Also called as Clarkson’s disease
Epidemiology:
- Usually seen in middle aged adults
- Equal M:F ratio
Etiology:
- Monoclonal gammopathy is noted in many patients
Pathogenesis:
Various mechanisms of capillary leak include
- Increased hydrostatic pressure: Heart failure, renal failure, portal hypertension, DVT etc
- Decreased capillary oncostatic pressure: Hypoalbuminemia due to decreased production or increased loss
- Increased capillary permeability: Sepsis, SIRS, acute pancreatitis, anaphylaxis, snake bite, infections ( dengue hemorrhagic fever, brucellosis, hantavirus cardiopulmonary syndrome, Covid 19 infection), Covid-19 vaccination)
Mechanisms suspected in idiopathic capillary leak syndrome:
- Paraprotein induced endothelial damage
- Increased levels of VEGF and angiopoietin 2, which leads to
- Disruption of endothelial cell junctions
- Cell retraction
- Endothelial cell apoptosis
- Increased IL-2
Clinical features:
- Attacks of capillary leak, which occurs in 3 phases (Frequency and severity differs in patients)
- Prodromal phase- Oligo-anuria, fatigue, edema, syncope, abdominal pain, nausea, myalgia of extremities, polydypsia, sudden increase in body weight
- Extravasation phase-
- 1-4 days after prodromal phase- hypotension (SBP <90mmHg), hemoconcentration, hypoalbuminemia
- Generalized edema, ascites, bilateral pleural effusion, cerebral edema and encephalopathy
- Systemic hypoperfusion: Cool extremities, restlessness, anuria, lactic acidosis
- Median duration- 3.8 days
- Recovery phase- Extravasated fluid returns back to intravascular space
Complications:
- Inadequate fluid rescuscitation can lead to stroke and ischemic hepatitis
- Renal failure due to
- Ischemic related acute tubular necrosis
- Rhabdomyolysis related renal failure
- Compartment syndrome with rhabdomyolysis
- Pulmonary edema
Investigations:
- S. Immunoglobulin levels, S. Protein electrophoresis, S. Free light chain assay
Criteria for diagnosis:
- Idiopathic capillary leak syndrome is a diagnosis of exclusion
- 1 or more episodes of following (in absence of an identifiable alternative cause)
- Intravscualrhypovolemia
- Generalised edema
- Hypotension
- Hemoconcentration
- Hypoalbuminemia
- Monoclonal gammopathy supports diagnosis, but is not required
Differential diagnosis:
- Severe sepsis/ septic shock
- Toxic shock syndrome
- Anaphylaxis
- Systemic mastocytosis
- Autoimmune diseases
- Drug reactions- IL2, G-CSF, INF alpha, gemcitabine, sirolimus, acitretin
- Hereditary angioedema
- Engraftment syndrome
- Differentiation syndrome
- Ovarian hyperstimulation syndrome
- Viral hemorrhagic fever
- HLH
- Snake bite evenomation
- Ricin poisoning
- Schnitzler syndrome- Chronic urticarial rash, IgM monoclonal gammopathy, fever, arthralgia, bone pain, and lymphadenopathy
Treatment:
- Treatment of shock
- Intubation/ mechanical ventilation if required
- Adequate fluid resuscitation with crystalloids/ colloids (Albumin, FFP etc)
- Ionotropes if required
- ECMO if required
- Red cell transfusions
- Medications which have been tried
- IVIg
- Terbutaline and IV aminophylline
- Infliximab
- Bevacizumab (AntiVEGF antibody)
Prevention: Agents which have been used include
- Monthly IVIg (400mg-2gm/Kg)
- Corticosteroids
- Terbutaline with theophylline
Figures:
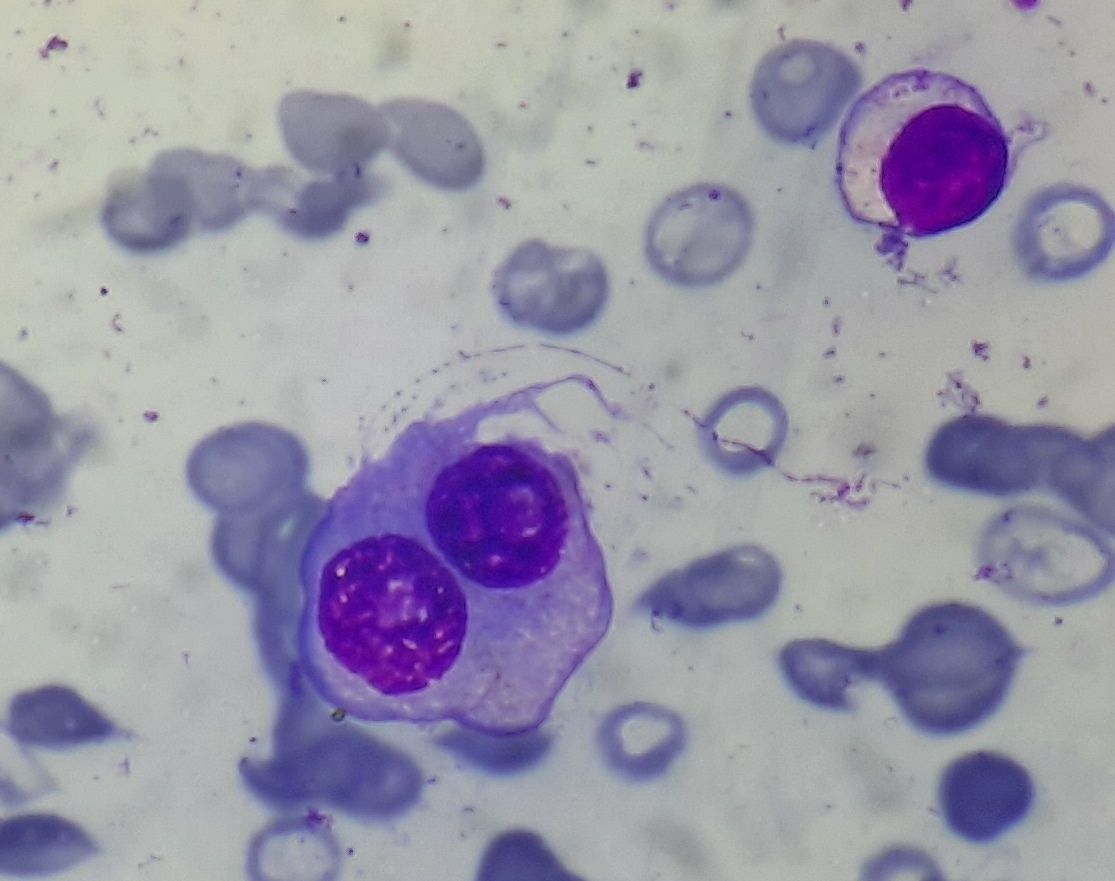
Figure 6.7.1- Multiple myeloma- Abnormal plasma cells
Recent advances:
Thromboprophylaxis in patients receiving treatment for multiple myeloma
There is limited data on use of DOACs as thromboprophylaxis in patients with multiple myeloma. In a study by Katrina Piedra, which involved 305 newly diagnosed myeloma patients, the incidence of VTE in patients receiving carfilzomib, lenalidomide, dexamethasone + aspirin, bortezomib, lenalidomide, dexamethasone with Aspirin and carfilzomib, lenalidomide, dexamethasone + rivaroxaban were 16·1%, 4·8%, and 4·8%, respectively. This shows risk of VTE is higher in KRD induction compared to RVD induction. This higher risk can be mitigated with use of low dose rivaroxaban.
https://doi.org/10.1111/bjh.17772
Novel PBX1-FOXM1 axis detected in chr1q-amp high risk myeloma
Clinical multi-omics study has showed that in patients with chr1q-amp associated high risk myeloma, there is involvement of core PBX1-FOXM1 regulatory axis. In view of availability of PBX1 inhibitor such as T417 and thiostrepton, these findings are said to be clinically significant. Chr1q-amp is a major copy number aberration (CNA) which confers adverse prognosis in several types of cancers. The technology used in identifying such pathways is called “systems medicine approach”.
https://doi.org/10.1182/blood.2021014391
PIM2 kinase has pivotal role in plasmablast generation
Differentiation of B cells into plasmablasts and then plasma cells involves extensive cell reprogramming. By using specific inhibition strategies (including a novel morpholino RNA antisense approach) researchers have found novel PIM2 kinase, which is highly expressed in malignant plasma cells. Experimental studies have also showed that pan-PIM inhibitors are useful in reducing proliferation of plasma cells. PIM2 may be used as therapeutic target in multiple myeloma.
https://doi.org/10.1182/blood.2021014011
Impact of maintenance therapy post autologous stem cell transplantation for multiple myeloma in early and delayed transplant
ASCT within 12 months of diagnosis is called early ASCT, whereas after 12 months is called as late ASCT. Most common maintenance approach was an IMiD (61%), followed by a PI (31%). PFS was superior with maintenance for early ASCT vs. for delayed ASCT. OS from diagnosis was also better for the whole cohort with maintenance therapy compared to patients not receiving maintenance therapy.
https://doi.org/10.1038/s41409-022-01631-8
Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma
Among 357 patients in the RVD-alone group and 365 in the transplantation group, at a median follow-up of 76.0 months, 328 events of disease progression or death occurred. The risk was 53% higher in the RVD-alone group than in the transplantation group. 5-year survival was 79.2% and 80.7% respectively.
https://doi.org/10.1056/NEJMoa2204925
Teclistamab in Relapsed or Refractory Multiple Myeloma
Teclistamab is a T-cell–redirecting bispecific antibody that targets both CD3 expressed on the surface of T cells and B-cell maturation antigen expressed on the surface of myeloma cells. Patients with relapsed/ refractory disease received a weekly subcutaneous injection of teclistamab (at a dose of 1.5 mg per kilogram of body weight) after receiving step-up doses of 0.06 mg and 0.3 mg per kilogram. With a median follow-up of 14.1 months, the overall response rate was 63.0%.
https://doi.org/10.1056/NEJMoa2203478
GPRC5D-Targeted CAR T Cells for Myeloma
G protein–coupled receptor, class C, group 5, member D (GPRC5D) has been identified as an immunotherapeutic target in multiple myeloma. In a recent study GPRC5D-targeted CAR T-cell therapy (MCARH109) was administered to patients with heavily pretreated multiple myeloma, including patients with relapse after BCMA CAR T-cell therapy. A response was reported in 71% of the patients in the entire cohort.
https://doi.org/10.1056/NEJMoa2209900
MRD after ASCR for patients with myeloma
Data from a large phase III trial (Myeloma XI) were examined to determine the relationship between MRD status, progression-free survival (PFS), and overall survival (OS) in post-ASCT patients randomly assigned to lenalidomide maintenance or no maintenance at 3 months after ASCT. MRD status was assessed by flow cytometry. MRD-negative status was associated with improved PFS and OS. Patients randomly assigned to lenalidomide maintenance were more likely to convert from being MRD-positive before maintenance random assignment to MRD-negative 6 months later (lenalidomide 30%, observation 17%).
https://doi.org/10.1200/JCO.21.02228
Identification of High-Risk Multiple Myeloma With a Plasma Cell Leukemia-Like Transcriptomic Profile
A molecular marker for primary plasma cell leukemia is currently lacking. In this study a transcriptomic classifier for PCL-like disease was bioinformatically constructed. Study concluded that primary plasma cell leukemia can be identified on the basis of a specific tumor transcriptome, which was also present in patients with high-risk newly detected multiple myeloma, despite not being clinically leukemic. Incorporating PCL-like status into current risk models in NDMM may improve prognostic accuracy.
https://doi.org/10.1200/JCO.21.01217
Talquetamab, a T-Cell–Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma
G protein–coupled receptor, family C, group 5, member D (GPRC5D) is an orphan receptor expressed in malignant plasma cells. Talquetamab, a bispecific antibody against CD3 and GPRC5D, redirects T cells to mediate killing of GPRC5D-expressing myeloma cells. In the prresent study talquetamab administered intravenously weekly or every other week (in doses from 0.5 to 180 μg per kilogram of body weight) or subcutaneously weekly, every other week, or monthly (5 to 1600 μg per kilogram) in patients who had heavily pretreated relapsed or refractory multiple myeloma. 232 patients had received talquetamab. Cytokine release syndrome, skin-related events, and dysgeusia were common with talquetamab treatment. A response rate of approximately 65% was observed.
https://doi.org/10.1056/NEJMoa2204591
Abnormal metaphase cytogenetics predicts venous thromboembolism in myeloma
The aim of this study was to develop a new risk prediction model for VTE in the context of modern antimyeloma therapy. A 5-component risk prediction tool, named the PRISM score, was developed, including the following variables: prior VTE, prior surgery, immunomodulatory drug use, abnormal metaphase cytogenetics, and Black race. The model stratified patients into low, intermediate, and high risk, with 12-month cumulative VTE incidence of 2.7%, 10.8%, and 36.5%, respectively. Risk of VTE increased significantly with increasing score.
https://doi.org/10.1182/blood.2022015727
Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents
B-cell maturation antigen (BCMA)–targeting therapies, including bispecific antibodies (BsAbs) and antibody-drug conjugates (ADCs), are promising treatments for multiple myeloma (MM), but disease may progress after their use. Cilta-cel, is an anti-BCMA chimeric antigen receptor T therapy. In the present study single cilta-cel infusion was given after lymphodepletion. Overall response rate was 60.0%. Median duration of response and progression-free survival were 11.5and 9.1 months, respectively.
https://doi.org/10.1182/blood.2022015526
Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma
Idecabtagene vicleucel (ide-cel) is a B-cell maturation antigen–directed chimeric antigen receptor (CAR) T-cell therapy. Present study involved adults with relapsed and refractory multiple myeloma who had received two to four regimens previously. They were randomly assigned to receive either ide-cel (dose range, 150×106 to 450×106 CAR-positive T cells) or one of five standard regimens. Ide-cel therapy significantly prolonged progression-free survival and improved response as compared with standard regimens.
https://doi.org/10.1056/NEJMoa2213614
Elotuzumab Plus Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma: ELOQUENT-3 Trial
Patients with RRMM who had received ≥ 2 prior lines of therapy, with disease refractory to last therapy and either refractory or relapsed and refractory to lenalidomide and a PI were randomly assigned (1:1) to receive EPd or Pd. EPd demonstrated a statistically significant improvement in OS versus Pd.
https://doi.org/10.1200/JCO.21.02815
Ciltacabtagene Autoleucel, an Anti–B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE Study.
Present study presents updated results 2 years after last patient in CARTITUDE Study. At a median follow up of 27.7 months, the overall response rate was 97.9% 27-month PFS and OS rates were 54.9% and 70.4%, respectively. Duration of response, PFS, and/or OS were shorter in patients with high-risk cytogenetics, International Staging System stage III, high tumor burden, or plasmacytomas.
https://doi.org/10.1200/JCO.22.00842
Bendamustine with pomalidomide and dexamethasone in relapsed/refractory multiple myeloma
In this phase II study, patients received bendamustine 120 mg/m2day 1, pomalidomide 3 mg days 1–21, and dexamethasone 40 mg days 1, 8, 11, 22. Regimen given for a maximum of six cycles. An ORR of 57.6% was achieved. At a median follow-up of 8.6 months, the median PFS and OS were 6.2 and 9.7 months respectively. Toxicity was manageable.
https://doi.org/10.1111/bjh.18200
Daratumumab: Results from the Canadian Myeloma Research Group Database
This study aimed to evaluate the outcomes of dara-containing regimens in the Canadian real-world setting among relapsed and refractory MM available within the national Canadian Myeloma Research Group Database. A total of 583 MM patients who received dara-based therapy in second-line or later treatment were included. After a median follow-up of 17.5 months, the median progression-free survival (PFS) and overall survival (OS) for the entire cohort were 13.1 and 32.9 months, respectively. The addition of bortezomib, lenalidomide or pomalidomide to dara resulted in an improved median PFS and OS of 8.3 and 26.2 months; 26.8 and 43.0 months; and 9.7 and 31.4 months respectively.
https://doi.org/10.1111/bjh.18172
Predictors of early mortality in multiple myeloma
Present study investigated early mortality in a prospective cohort study of all patients with newly diagnosed myeloma registered on the Australian and New Zealand Myeloma and Related Diseases Registry at 36 institutions between July 2011 and March 2020. Variables that were independent predictors of early mortality were age, Eastern Cooperative Oncology Group performance status, serum albumin, cardiac disease and International Staging System.
https://doi.org/10.1111/bjh.18324
Belantamab mafodotin therapy for relapsed/refractory multiple myeloma
Belantamab mafodotin, an immuno-conjugate targeting B-cell maturation antigen and was recently approved for heavily pretreated relapsed/refractory multiple myeloma patients. This real world data showed response rate of 45.5%. Median progression-free survival was 4.7 months.
https://doi.org/10.1111/bjh.18479
Ultra-High-Risk Multiple Myeloma: Induction with Daratumumab, Cyclophosphamide, Bortezomib, Lenalidomide and Dexamethasone
The OPTIMUM phase II trial assessed Dara-CVRd treatment in high-risk multiple myeloma patients before and after autologous stem-cell transplant (ASCT), comparing outcomes with the MyeXI trial. UHiR patients received Dara-CVRd induction, ASCT, and Dara-VRd consolidation. Comparison showed OPTIMUM's superiority (99.5% probability) in 18-month progression-free survival (PFS18m) over MyeXI. At 30 months, OPTIMUM demonstrated improved PFS (77% vs. 39.8%) and overall survival (OS) (83.5% vs. 73.5%). Extended Dara-VRd post-ASCT consolidation was well-tolerated.
DOI: 10.1200/JCO.22.02567
Role of PET/CT after autologous stem cell transplantation in myeloma patients
This phase II study evaluated the impact of 18F-Fluorodeoxyglucose PET positivity after autologous stem cell transplantation (ASCT) in multiple myeloma patients. PET-positive patients achieving PET negativity after treatment displayed comparable outcomes to initially PET-negative patients. The study screened 159 post-ASCT patients with very good partial response (VGPR) or better. Of PET-positive patients, 57% were MRD-negative, indicating complementary response assessment. Carfilzomib-lenalidomide-dexamethasone (KRd) consolidation converted 33% of PET-positive patients to PET negativity, with better success among MRD-negative patients. Overall, PET effectively identified residual disease after ASCT, and KRd consolidation altered PET status in a subset of patients.
https://doi.org/10.1038/s41375-023-01998-7
Mezigdomide plus Dexamethasone in Relapsed and Refractory Multiple Myeloma
This study focused on evaluating mezigdomide, a novel cereblon E3 ubiquitin ligase modulator, in combination with dexamethasone for patients with relapsed and refractory multiple myeloma. In the phase 1 part of the study, 77 patients were enrolled, and the recommended phase 2 dose was determined to be 1.0 mg of mezigdomide given once daily with dexamethasone for 21 days, followed by 7 days off, in each 28-day cycle. In the phase 2 portion, 101 patients with triple-class–refractory multiple myeloma were treated. Common adverse events included neutropenia (77%) and infections (65%). An overall response rate of 41% was observed, with a median duration of response of 7.6 months and a median progression-free survival of 4.4 months. This combination demonstrated promising efficacy in heavily pretreated multiple myeloma patients, with manageable adverse effects primarily related to bone marrow suppression.
https://doi.org/10.1056/NEJMoa2303194
Weekly carfilzomib 70 mg/m2 and dexamethasone with or without cyclophosphamide in relapsed and/or refractory multiple myeloma patients
In this randomized phase II study for relapsed/refractory multiple myeloma (RRMM) patients with 1-3 prior lines of treatment, the combination of weekly carfilzomib, cyclophosphamide, and dexamethasone (KCd) was compared to carfilzomib and dexamethasone (Kd). Out of 197 patients, 97 received KCd, and 100 received Kd. Median progression-free survival (PFS) was similar at 19.1 months for KCd and 16.6 months for Kd. However, in a post hoc analysis of lenalidomide-refractory patients, the addition of cyclophosphamide to Kd significantly improved PFS to 18.4 months versus 11.3 months. Both groups achieved similar overall response rates and complete response rates (around 70% and 20%, respectively). Safety profiles were manageable, but severe infections were more common with the KCd combination. In conclusion, KCd did not improve outcomes overall but showed promise for lenalidomide-refractory patients in this RRMM study.
https://doi.org/10.3324/haematol.2022.282490
Single-agent belantamab mafodotin versus pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma
Belantamab is a humanised anti-BCMA afucosylated monoclonal antibody. In the DREAMM-3 phase 3 study for relapsed or refractory multiple myeloma, belantamab mafodotin was compared to the standard of care, pomalidomide-dexamethasone. While belantamab mafodotin showed no statistically significant improvement in progression-free survival, it demonstrated a manageable safety profile. Thrombocytopenia and anaemia were the most common grade 3-4 adverse events for belantamab mafodotin, while neutropenia and anaemia were predominant for pomalidomide-dexamethasone. Serious adverse events occurred in both groups, but there were no treatment-related deaths with belantamab mafodotin. Further research is ongoing to explore belantamab mafodotin in combination regimens for multiple myeloma.
https://doi.org/10.1016/S2352-3026(23)00243-0
Efficacy and safety of isatuximab plus bortezomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma ineligible/with no immediate intent for autologous stem cell transplantation
This Phase 1b study assessed the combination of isatuximab with bortezomib-lenalidomide-dexamethasone (Isa-VRd) in newly diagnosed multiple myeloma (NDMM) patients ineligible forASCT. Isatuximab is a chimeric monoclonal antibody targeted against surface CD38 glycoproteins. Among 73 patients, the overall response rate was 98.6%, with 56.3% achieving a complete response or better. Minimal residual disease negativity (10^-5 sensitivity) was attained by 50.7% of patients. Grade ≥3 treatment-emergent adverse events occurred in 79.5% of patients, but discontinuation of study treatment due to adverse events was observed in 19.2% of patients.
https://doi.org/10.1038/s41375-023-01936-7
Post-transplant relapse prediction score in multiple myeloma
This study aimed to predict early relapse (ER) following Autologous Hematopoietic Cell Transplantation (AHCT) and developed a novel scoring system based on various factors. A total of 14,367 AHCT-1 patients were transplanted between 2014 and 2019, with training and validation cohorts. The scoring system assigned points for factors including ISS stages, disease status, and Karnofsky performance score. The risk score distribution ranged from 0 to ≥4 points. The lowest risk group had a 12-month progression-free survival (PFS-12) of 91.7%, while the highest risk group had a PFS-12 of 57.1%.
https://doi.org/10.1038/s41409-023-01999-1
Pomalidomide and dexamethasone until progression after first salvage therapy in multiple myeloma
This study investigated the use of pomalidomide as a maintenance therapy in multiple myeloma patients in their first relapse. Patients received pomalidomide, cyclophosphamide, and dexamethasone salvage therapy, followed by transplantation or consolidation cycles. Out of 100 patients, 75 reached therapy and showed a median treatment duration of 23.7 months. One third of the patients experienced an improved treatment response, with median progression-free survival of 33.2 months. Adverse events, mainly hematological, were observed, but long-term administration of pomalidomide and dexamethasone was feasible and demonstrated the potential for response improvement in some patients.
https://doi.org/10.1111/bjh.18772
Lenalidomide and dexamethasone maintenance with or without ixazomib, tailored by residual disease status in myeloma
In a study from November 2014 to May 2017, 332 patients treated with bortezomib, lenalidomide, and dexamethasone (VRD) were randomized for maintenance therapy with lenalidomide and dexamethasone (RD) or RD plus ixazomib (IRD) after autologous stem cell transplant and VRD consolidation. After a median follow-up of 69 months, progression-free survival (PFS) was similar between RD and IRD groups (61.3% vs. 55.6% at 6 years), supporting the efficacy of RD maintenance. Discontinuation of maintenance in patients with negative measurable residual disease (MRD) at 2 years showed low progression rates (17.2% at 4 years), even in those with high-risk features.
https://doi.org/10.1182/blood.2022019531
Minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma (MASTER)
The MASTER trial investigated the use of daratumumab, carfilzomib, lenalidomide, and dexamethasone (Dara-KRd) therapy in newly diagnosed multiple myeloma patients, where minimal residual disease (MRD) status was utilized to modulate treatment duration and cessation. The study included 123 participants, and after the induction phase followed by autologous stem-cell transplantation and consolidation with Dara-KRd, 81% achieved MRD negativity. Of those, 71% reached MRD-SURE and treatment cessation. Progression-free survival at 36 months varied based on the number of high-risk chromosome abnormalities (HRCAs). While participants without HRCAs had an 88% progression-free survival, those with two or more HRCAs had a 50% progression-free survival. Notably, 61 participants remain free of therapy and MRD-negative as of February 7, 2023.
https://doi.org/10.1016/S2352-3026(23)00236-3
Thromboprophylaxis in patients with multiple myeloma
The meta-analysis comparing direct oral anticoagulants (DOACs) with aspirin for primary thromboprophylaxis in multiple myeloma patients undergoing anti-myeloma therapy (n=1026) found that DOACs were associated with a significantly lower incidence of venous thromboembolism (VTE) . Major bleeding, clinically relevant non-major bleeding, and minor bleeding event rates did not significantly differ between DOACs and aspirin. While DOACs may be preferable for MM-related thrombosis prevention, further research is needed to establish optimal thromboprophylaxis strategies, considering heterogeneous baseline characteristics and potential bias from observational studies.
https://doi.org/10.1111/bjh.19017
Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma
The GMMG-CONCEPT trial investigated the combination of isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in patients with newly diagnosed multiple myeloma (NDMM) with exclusively high-risk disease. The trial enrolled both transplant-eligible (TE) and transplant-noneligible (TNE) patients with high-risk cytogenetic aberrations (HRCAs). The study aimed to induce minimal residual disease (MRD) negativity. The interim analysis (IA) included 125 patients with high-risk NDMM. The trial met its primary endpoint, with MRD negativity rates after consolidation of 67.7% in TE patients and 54.2% in TNE patients. MRD negativity was sustained for ≥1 year in 62.6% of patients. With a median follow-up of 44 months for TE patients and 33 months for TNE patients, median progression-free survival (PFS) was not reached in either arm. The combination of Isa-KRd demonstrated efficacy in inducing sustainable MRD negativity in patients with high-risk NDMM, regardless of transplant status, and showed promising PFS outcomes.
https://doi.org/10.1200/JCO.23.01696
Elotuzumab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma
The real-world study involving 200 cases of relapsed/refractory multiple myeloma (RRMM) treated with the combination of elotuzumab, pomalidomide, and dexamethasone (EloPd) in 35 Italian centers outside of clinical trials provides insights into the treatment's efficacy and safety. The overall response rate was 55.4%, consistent with the pivotal trial results. The toxicity profile in the real-world cohort was similar to the ELOQUENT-3 trial, with no significant differences between younger and older patients.
https://doi.org/10.3324/haematol.2023.283251
Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma
In a phase 3 trial involving 709 transplantation-eligible patients with newly diagnosed multiple myeloma, the efficacy of subcutaneous daratumumab combined with bortezomib, lenalidomide, and dexamethasone (D-VRd) was compared to VRd alone. The D-VRd group showed a lower risk of disease progression or death, with an estimated 84.3% progression-free survival at 48 months, compared to 67.7% in the VRd group. The D-VRd group also exhibited higher rates of complete response (87.9% vs. 70.1%) and minimal residual disease (MRD)-negative status (75.2% vs. 47.5%). The addition of subcutaneous daratumumab demonstrated a significant benefit in progression-free survival for transplantation-eligible patients with newly diagnosed multiple myeloma.
https://doi.org/10.1056/NEJMoa2312054
Idecabtagene vicleucel chimeric antigen receptor T-cell therapy for relapsed/refractory multiple myeloma with renal impairment
Idecabtagene vicleucel (ide-cel) is a B-cell-maturation antigen (BCMA)-directed chimeric antigen receptor T cell therapy. Patients with relapsed multiple myeloma and renal impairment treated with ide-cel showed comparable safety and efficacy outcomes to those without renal impairment. While rates of cytokine release syndrome and neurotoxicity were similar between groups, patients with renal impairment had a higher incidence of short-term severe cytopenias. However, renal function did not deteriorate following CAR T-cell therapy, and response rates and survival outcomes were comparable between the two groups, highlighting the feasibility of ide-cel treatment in patients with renal impairment.
https://doi.org/10.3324/haematol.2023.283940
Melflufen plus dexamethasone and daratumumab or bortezomib in relapsed/refractory multiple myeloma
The ANCHOR study assessed melflufen (Melphalanflufenamide, alkylating peptide-drug conjugate) in combination with dexamethasone and daratumumab or bortezomib in triple-class refractory multiple myeloma (MM). No dose-limiting toxicities were observed, and thrombocytopenia and neutropenia were the most common grade ≥3 adverse events. In the daratumumab arm, the overall response rate was 73%, with a median progression-free survival of 12.9 months, while in the bortezomibarm, the overall response rate was 78%, with a median progression-free survival of 14.7 months. Based on these findings, melflufen 30 mg was determined as the recommended dose for future studies in relapsed/refractory MM when used with dexamethasone and daratumumab or bortezomib.
https://doi.org/10.3324/haematol.2023.283490
Best treatment strategy before autologous peripheral blood stem cell transplantation in POEMS syndrome
This study conducted in ten Italian centers evaluated the impact of prior therapies and mobilization regimen on outcomes in patients with POEMS syndrome undergoing autologous peripheral blood stem cell transplantation (aPBSCT) from 1998 to 2020. Patients were divided into three groups based on their treatment history before transplant. Regardless of prior treatment received, all 45 patients underwent aPBSCT after a high-dose melphalan conditioning regimen. The study found no statistically significant differences in responses, progression-free survival, overall survival, or transplant-related mortality among the three groups, suggesting that pre-treatment and disease status at transplant did not affect major transplant outcomes in patients with POEMS syndrome.
https://doi.org/10.3324/haematol.2023.283719
CAR-T and BITE therapies in multiple myeloma patients with prior allogeneic transplantation
In a cohort of 33 multiple myeloma (MM) patients with prior allogeneic hematopoietic cell transplantation (allo-HCT), chimeric antigen receptor T-cell (CAR-T) therapy (n = 24) demonstrated an overall response rate (ORR) of 92%, with 67% achieving a stringent complete response (CR), and 73% achieving minimal residual disease (MRD) negativity. Bispecific T-cell engagers (BITE) therapy (n = 9) resulted in an ORR of 44%, with 44% achieving a CR, and 44% achieving MRD negativity. Safety analysis revealed grade ≥3 adverse events (AEs) in 92% of CAR-T and 56% of BITE recipients, with cytokine release syndrome (CRS) occurring in 83% of CAR-T and 78% of BITE recipients. Notably, no exacerbation of graft-versus-host disease occurred except in one BITE recipient.
https://doi.org/10.1111/bjh.19244
Teclistamab in Relapsed/ Refractory multiple myeloma
Teclistamab, a B-cell maturation antigen (BCMA) × CD3 directed bispecific antibody. In a retrospective analysis of 123 patients with relapsed and refractory multiple myeloma (RRMM) treated with teclistamab at 18 German centers, outcomes were assessed to determine real-world efficacy and tolerability. Most patients had triple-class or penta-drug refractory disease, with a subset previously treated with BCMA-directed therapies. The overall response rate (ORR) was 59.3% with a median progression-free survival (PFS) of 8.7 months. Subgroup analyses revealed lower ORR and PFS in patients with extramedullary disease, ISS 3, and those previously treated with idecabtagenevicleucel (ide-cel) CAR-T cell therapy, although duration of response in ide-celpretreated patients was comparable to anti-BCMA naive patients. Adverse events primarily included infections and grade ≥3 cytopenias.
https://doi.org/10.1038/s41375-024-02154-5
Artificial intelligence in prognosticating multiple myeloma
This study aimed to decipher and predict the diverse outcomes seen in newly diagnosed multiple myeloma (NDMM) patients by analyzing clinical, genomic, and therapeutic data from 1,933 individuals. Through this analysis, the researchers identified 12 distinct groups based on genomic drivers, surpassing previous classifications. They developed a predictive model called IRMMa, integrating clinical, genomic, and treatment variables, which outperformed existing prognostic models in accuracy. Key genomic features, such as 1q21 gain/amp and TP53 loss, contributed to the model's precision. The study underscores the importance of personalized therapeutic decisions in NDMM based on individualized risk assessment, potentially optimizing treatment outcomes.
https://doi.org/10.1200/JCO.23.01277
Autologous-allogeneic versus autologous tandem stem cell transplantation and maintenance therapy with thalidomide for multiple myeloma patients
This clinical trial compared autologous-allogeneic tandem stem cell transplantation (alloTSCT) to autologous tandem transplantation (autoTSCT) followed by thalidomide maintenance therapy in patients with multiple myeloma. Among the 217 patients enrolled, 178 underwent a second transplant, with alloTSCT showing a non-significant trend towards better progression-free survival (PFS) at 4 years (47% vs. 35%, P=0.26) and a more substantial difference at 8 years (P=0.10). AlloTSCT significantly reduced relapse rates at 4 years (40% vs. 63%, P=0.04) and at 8 years (44% vs. 77%, P=0.002). However, overall survival at 4 and 8 years was similar between both groups (66% vs. 66% at 4 years; 52% vs. 50% at 8 years, P=0.91 and P=0.87, respectively).
https://doi.org/10.3324/haematol.2023.282920
ChatGPT as an educational resource for patients with multiple myeloma
This study evaluated ChatGPT's potential as an educational tool for multiple myeloma (MM) patients by assessing its responses to a 21-question questionnaire. ChatGPT demonstrated a high accuracy rate (95%), with no harmful responses, though one treatment-related question was flagged for incompleteness. Simplifying medical terminology and ensuring up-to-date, comprehensive responses were noted as areas for improvement. Overall, ChatGPT shows promise as a supplementary educational resource, enhancing patient understanding while emphasizing the need for ongoing refinements and human medical expertise.
https://doi.org/10.1002/ajh.27318
Linvoseltamab for Treatment of Relapsed/Refractory Multiple Myeloma
In a phase I/II trial, linvoseltamab, a B-cell maturation antigen × CD3 bispecific antibody, was evaluated for safety and efficacy in relapsed/refractory multiple myeloma (RRMM) patients. Among 117 patients treated with the 200 mg dose, the overall response rate (ORR) was 71%, with 50% achieving a complete response or better. The median duration of response was 29.4 months. The most common adverse events were cytokine release syndrome, neutropenia, and infections, but these events were manageable. Linvoseltamab 200 mg showed deep and durable responses with an acceptable safety profile in heavily pretreated RRMM patients.
https://doi.org/10.1200/JCO.24.01
Pembrolizumab and low-dose, single-fraction radiotherapy for patients with relapsed or refractory multiple myeloma
This phase 2 trial at Winship Cancer Institute evaluated the safety and activity of pembrolizumab combined with low-dose radiotherapy (8 Gy/1 fx) in 25 patients with relapsed/refractory multiple myeloma. The primary outcome was acceptable toxicity, with no grade 4 or 5 adverse events reported. At 3 months, 32% of patients showed treatment benefits, including partial and complete responses, with no severe radiation-related toxicity.
https://doi.org/10.1016/S2352-3026(24)00105-4
Belantamab Mafodotin, Bortezomib, and Dexamethasone for Multiple Myeloma
This phase 3 trial compared belantamab mafodotin, bortezomib, and dexamethasone (BVd) versus daratumumab, bortezomib, and dexamethasone (DVd) in relapsed or refractory multiple myeloma. BVd significantly improved progression-free survival (36.6 vs. 13.4 months) and overall survival at 18 months (84% vs. 73%). BVd also led to higher rates of complete response and minimal residual disease negativity (25% vs. 10%). However, BVd was associated with more grade 3 or higher adverse events (95% vs. 78%), particularly ocular events. BVd demonstrated strong efficacy but with notable safety concerns.
https://doi.org/10.1056/NEJMoa2405090
Curative Strategy for High-Risk Smoldering Myeloma
This study evaluated a curative approach for high-risk smoldering myeloma using carfilzomib, lenalidomide, and dexamethasone (KRd), followed by autologous stem-cell transplantation (ASCT), consolidation, and maintenance. Among 90 patients, 62% achieved undetectable measurable residual disease (uMRD) post-ASCT, and 31% maintained uMRD at 4 years. The overall survival rate at 70 months was 92%, with 5 patients progressing to multiple myeloma. Neutropenia and infections were common adverse events. These findings suggest a promising outcome for early aggressive treatment in high-risk smoldering myeloma.
https://doi.org/10.1200/JCO.23.02771
Efficacy and safety of teclistamab in patients with relapsed/refractory multiple myeloma after BCMA-targeting therapies
Teclistamab, a BCMA-directed bispecific antibody, showed promising efficacy and safety in heavily pretreated relapsed/refractory multiple myeloma (R/RMM) patients with prior anti-BCMA therapy, according to the MajesTEC-1 trial cohort. Among 40 patients with a median of six prior treatment lines, the overall response rate was 52.5%, with 47.5% achieving very good partial response or better. The median duration of response, progression-free survival, and overall survival were 14.8, 4.5, and 15.5 months, respectively. Common treatment-emergent adverse events included neutropenia, infections, and cytokine release syndrome, with infections being the most frequent grade ≥3 adverse events. These results support teclistamab as an effective option for this challenging patient population.
https://doi.org/10.1182/blood.2023023616
Anti-BCMA/GPRC5D bispecific CAR T cells in patients with relapsed or refractory multiple myeloma
This phase 1 trial evaluated anti-BCMA/GPRC5D bispecific CAR T cells in 21 patients with relapsed/refractory multiple myeloma, identifying 2.0 × 10⁶ CAR T cells per kg as the maximum tolerated dose. At this dose, no severe organ toxicities or grade ≥3 ICANS occurred, and cytokine release syndrome was mild (grades 1–2). The overall response rate was 92% at the MTD, with 75% achieving complete response or better and 81% attaining measurable residual disease negativity. These findings highlight the promising safety and efficacy of bispecific CAR T cells as a potential new treatment option for this challenging patient population.
https://doi.org/10.1016/S2352-3026(24)00176-5
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.