howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Primary CNS Lymphoma
Following must be excluded before making diagnosis:
- Lymphoma of dura
- Intravascular large B cell lymphoma
- Evidence of systemic lymphoma
- Immunodeficiency associated lymphoma
Note: Primary ocular lymphoma is considered as variant of primary CNS lymphoma
Epidemiology:
- Less than 1% of all NHL
- 2-3% of all brain tumors
- Median age- 60 years
- Slight male preponderance
Sites:
- 60%- Supratentorial
- 20-40%- Multiple lesions
- 20%- Intraocular lesions
Histological subtypes
- DLBCL-95% cases- Usually activated B cell type
- Peripheral T cell lymphoma
- Low grade lymphoma
- Burkitt lymphoma
Clinical features:
- Focal neurological deficits (50-80%)
- Neuropsychiatric symptoms (20-30%) - Behavioral changes, memory and language impairment, seizures, etc
- Leptomeningeal involvement- Headache, cranial nerve palsies
- Raised intracranial pressure
Investigations:
- Stereotactic/ open biopsy: (Steroids must be withheld for some before biopsy, as steroids interfere with pathological diagnosis. If lesion has resolved, serial MRIs may be needed to document regrowth of tumor, following which urgent biopsy can be done)
- Diffuse growth pattern
- Tumor cells are present in perivascular space
- Tumor cells resemble centroblasts
- There can be infiltration of reactive small lymphocytes, macrophages, activated microgial cells and reactive astrocytes.
- Large areas of necrosis with foamy histiocytes may be seen. (Especially if steroids have been administered)
- CSF cytology and flow cytometry
- This can be used for biopsy is not possible
- 3-10ml fluid must be used and sample must be processed rapidly, to increase the chances of diagnosis.
- 16% cases have leptomeningeal involvement
- Slit lamp examination
- 20% PCNSL have intraocular involvement at diagnosis
- Contrast enhanced MRI of brain
- Radiological investigation of choice at diagnosis and also for response assessment.
- Radiological differential diagnosis include- neurosarcoidosis, multiple sclerosis, glioblastoma, and vasculitis.
- Whole body PET CT- Needed to rule out systemic involvement
Prognosis:
- Usually, poor
- Median survival after surgery alone- 1-4 months
- Whole brain radiation- Response rate is 90%, but median survival with this modality is 12 months and also patients develop severe cognitive impairment.
- With HD-MTX based regimens, 5-year OS is 30% to 40%.
- Following 5 factors are associated with poor prognosis
- Age >60 years
- PS >2
- Elevated LDH
- High CSF fluid protein concentration
- Tumor location within the deep regions of brain
Number of factors | 2 year overall survival |
0 | 80% |
1-4 | 48% |
5 | 15% |
Pretreatment work up:
- History
- B-Symptoms
- Examination
- LN:
- Spleen:
- Ophthalmoscopy:
- WHO P. S.
- BSA
- IHC
- Grade
- BMA and Bx
- Contrast enhanced MRI of brain
- CT (CAP)/ PET
- Stage
- Hemoglobin
- TLC, DLC
- Platelet count
- LFT: Bili- T/D SGPT: SGOT: Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid:
- LDH
- HIV:
- HBsAg:
- HCV:
- UPT
- ECHO (If anthracyclines planned) LVEF- %
- LP if safe
- Spinal MRI, if symptomatic/ Positive CSF
- Testicular ultrasound
- Slit lamp examination of eyes for ocular involvement
Treatment Plan:
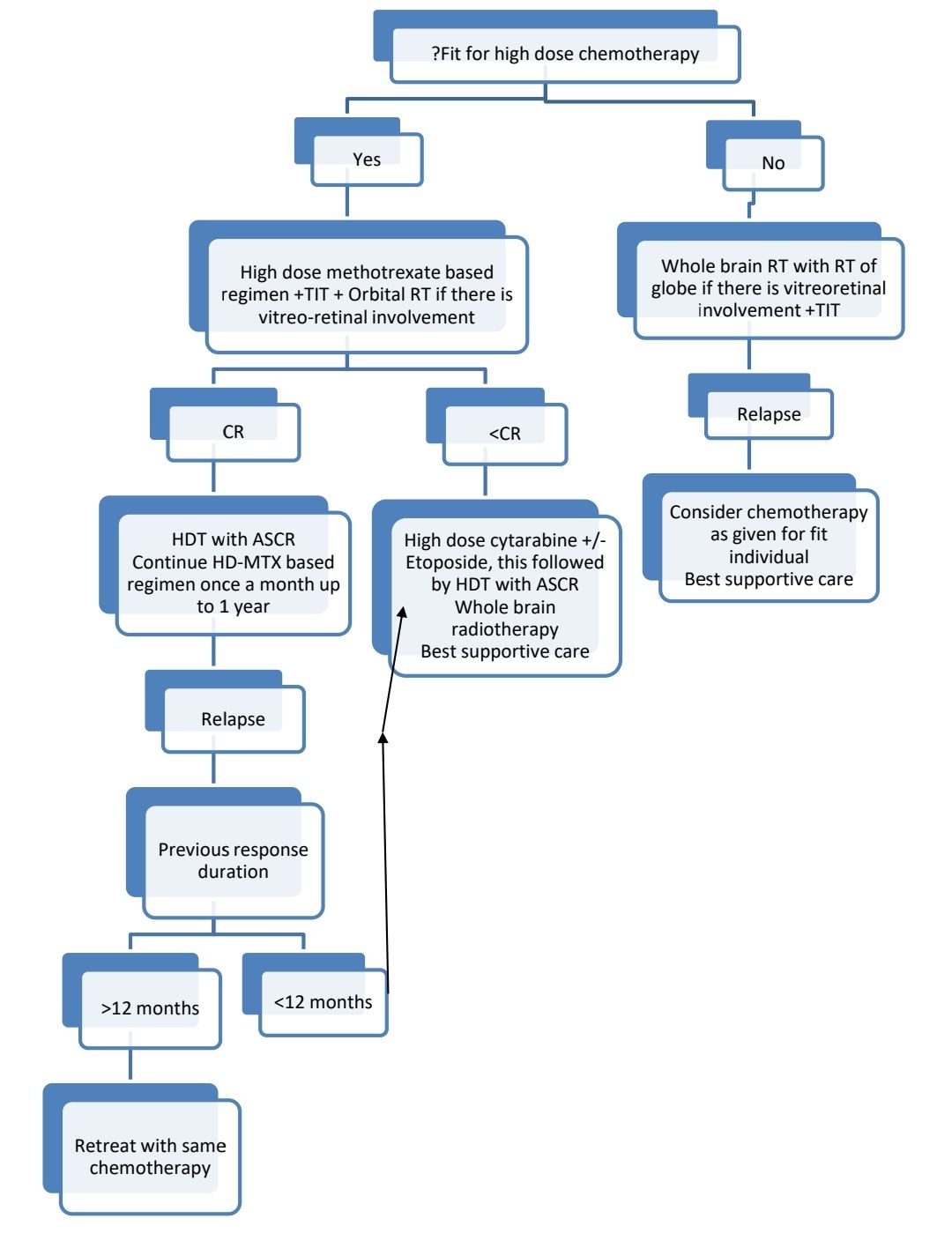
Response assessment:
Response | MRI Brain | Eye examination | CSF Cytology |
Complete remission (CR) | No contrast enhancement | Normal | Negative |
Unconfirmed complete remission (CRu) | No contrast enhancement Minimal abnormality | Normal | Negative |
Partial remission (PR) | 50% decrease in enhancing tumor | Minor retinal pigment epithelium abnormality | Negative |
Stable disease (SD) | <50% decrease and <25% increase in lesion | No new ocular disease | Persistent |
Progressive disease (PD) | >25% increase in lesion/ any new site of disease | New ocular disease | Recurrence/ new disease |
About Each Modality of Treatment:
- R- HD MTX for Primary CNS lymphoma
- Frequency: 14 days (Total 4 cycles)
- Protocol:
- Day 1:
- Hydration- ½ NS/ NS with 6 amp NaHCO3- 125ml/m2/hr till end of last Folinic acid (Start chemo after at least 12hrs of hydration)
- Measure urine pH after 6 hrs of hydration. Start chemo once urine pH is >7.
- Inj. Methotrexate- 500mg/m2 in 100ml NS over 15min
- Inj. Methotrexate- 3000mg/m2 (May be increased up to 8000mg/m2)- in 500ml NS over 3hrs
- Inj. Folinic acid- 30mg- IV- Bolus- 6 hrly for 6 doses- Start at 24hrs of starting methotrexate infusion.
- Inj. Methotrexate- 12.5mg- Intrathecal stat
- Day 8:
- Rituximab- 375mg/m2- As above
- Dose adjustments:
- ANC<1000/cmm or platelet count- <1,00,000/cmm- Delay cycle by 1 week
- Creatinine clearance (ml/min):
- 20-50- Give 50% of dose of methotrexate
- <20- Do not give methotrexate
- Bilirubin (mg/dL)
- 3-5 or SGPT/SGOT- >180- Give 75% dose of methotrexate
- >5- Do not give methotrexate
- If vitreo-retinal involvement- Orbital RT must be given
- Other HD-MTX based induction chemotherapy options (Each 4 cycles) include
- Rituximab + HD Methotrexate with high dose cytarabine
- Rituximab + HD methotrexate with high dose cytarabine and thiotepa (MATRix protocol). If this protocol is used, PBSC must be collected after 2 cycles of MATRix, as after thismobalizing stem cells is very difficult. This protocol is designed for extremely fit patients. It has shown clear survival advantage over other chemotherapy protocols.
- Rituximab + HD Methotrexate with temozolamide
Consolidation therapy
- Due to risk of early relapse, consolidation must be started within 6-8 weeks of the first day of final induction chemotherapy cycle.
- Patient’s performance score must be reassessed prior to starting consolidation.
- Response assessment with contrast enhanced MRI must be done 1-2 months after completion of consolidation therapy.
- Options of consolidation include
- Whole brain radiotherapy-
- 36 Gy- in 20 fractions
- Consider 9gy boost with a 1-2cm margin to residual enhancing lesion at the time of WBRT.
- Orbits should be shielded after 30gy (36 Gy, if previously documented intraocular disease)
- Sometimes it is associated with disabling neurocognitive impairment
- Avoid WBRT in patients aged >60 years due to high risk of neurocognitive impairment
- High dose therapy with Autologous stem cell rescue
- Thiotepa/ carmustine based conditioning protocols must be used
- Results with BEAM conditioning are disappointing
- This is better than WBRT
- Whole brain radiotherapy-
Follow up:
- Brain MRI- Once in 3 months for 2 years, then once in 6 months until 5 years, then annually
- If there was ocular involvement: Regular ophthalmoscopic evaluation
Related disorders:
- Primary intraocular lymphoma
- Intravitrial chemotherapy- MTX+ rituximab can achieve remission in proportion of patients, but 50% of these patients present with CNS relapse.
- Many have co-existing CNS disease
- Even those without CNS disease must be treated with systemic HD-MTX based chemotherapy with Rituximab. Treatment is similar to PCNSL.
Recent advances:
Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients Age 60 Years and Younger: Long-Term Results of the Randomized Phase II PRECIS Study
Patients were treated with high-dose methotrexate-based induction chemotherapy followed by whole-brain radiotherapy (WBRT) or high-dose chemotherapy (thiotepa-busulfan-cyclophosphamide) with autologous stem-cell transplantation Their 8-year event-free survival from random assignment was 67% and 39% in the ASCT and WBRT arms, respectively, with a significantly lower risk of relapse after ASCT. The 8-year overall survival was 69% and 65% in the ASCT and WBRT arms, respectively (not significant).
https://doi.org/10.1200/JCO.22.00491
Circulating Tumor DNA Profiling for Detection, Risk Stratification, and Classification of Brain Lymphomas
CNSL diagnosis often remains unconfirmed because of contraindications for invasive stereotactic biopsies. Therefore, improved biomarkers are needed to better stratify patients into risk groups, predict treatment response, and noninvasively identify CNSL. Hence present study explored the value of circulating tumor DNA (ctDNA) for early outcome prediction, measurable residual disease monitoring, and surgery-free CNSL identification by applying ultrasensitive targeted next-generation sequencing to a total of 306 tumor, plasma, and CSF specimens from 136 patients with brain cancers, including 92 patients with CNSL. Before therapy, ctDNA was detectable in 78% of plasma and 100% of CSF samples. Measurable residual disease detection by plasma ctDNA monitoring during treatment identified patients with particularly poor prognosis following curative-intent immunochemotherapy.
https://doi.org/10.1200/JCO.22.00826
Lenalidomide following whole-brain radiotherapy in patients with primary central nervous system lymphoma ineligible for intensive systemic therapy
This retrospective analysis explored the use of lenalidomide as a maintenance therapy following salvage whole-brain radiotherapy (WBRT) in patients with primary central nervous system lymphoma (PCNSL) who were not eligible for intensive systemic therapy. Fifteen patients received WBRT followed by lenalidomide, with a median duration of lenalidomide treatment of 18 months. The study cohort had a median age of 57 years, and the majority were male. The progression-free survival (PFS) and overall survival (OS) outcomes were higher compared to previous reports of WBRT or single-agent lenalidomide, with a 2-year PFS rate of 70%. The findings suggest a potential additive benefit of lenalidomide after WBRT and support its safety in this context.
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.