howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Red Blood Cell
Structure of RBC membrane:
- It is a biphospholipid protein complex with several proteins embedded within it.
- Lipids:
- Constitute 50-60% of membrane mass
- Glycolipids and cholesterol are intercalated between phospholipid bilayer.
- Unesterified cholesterol affects surface area of RBCs. It exists in free equilibrium with plasma cholesterol. When cholesterol levels are high in plasma, it accumulates within the RBC membrane leading to formation of target cells.
- Phospholipids in RBC membrane include cephalin, lecithin, sphingomyelin, phosphatidyl serine and phosphatidyl ethanolamine.
- Glycolipids- They include cerebrosides and gangliosides. They are responsible for some antigenic properties of RBCs, including blood groups such as A, B, H and P.
- Proteins: Usually identified by a number according to their separation on polyacrylamide gel electrophoresis.
- Integral proteins- Firmly entrenched in the lipid bilayer. They include
- Glycophorins- These are some blood group antigens
- Band 3 protein- Important in attaching skeletal protein network to the lipid bilayer.
- Peripheral proteins- They are present outside the lipid framework, on the cytoplasmic side of the membrane. They are responsible for 2 types of interactions. Horizontal interaction in lattice provides skeletal support for membrane lipid layer. Vertical interaction serves to attach the lattice network to the lipid layer of membrane. Peripheral proteins include
- Spectrin
- Actin
- Ankyrin (Band 2.1)
- Band 4.2
- Band 4.1
- Adducin
- Dematin
- Tropomyosin
- Integral proteins- Firmly entrenched in the lipid bilayer. They include
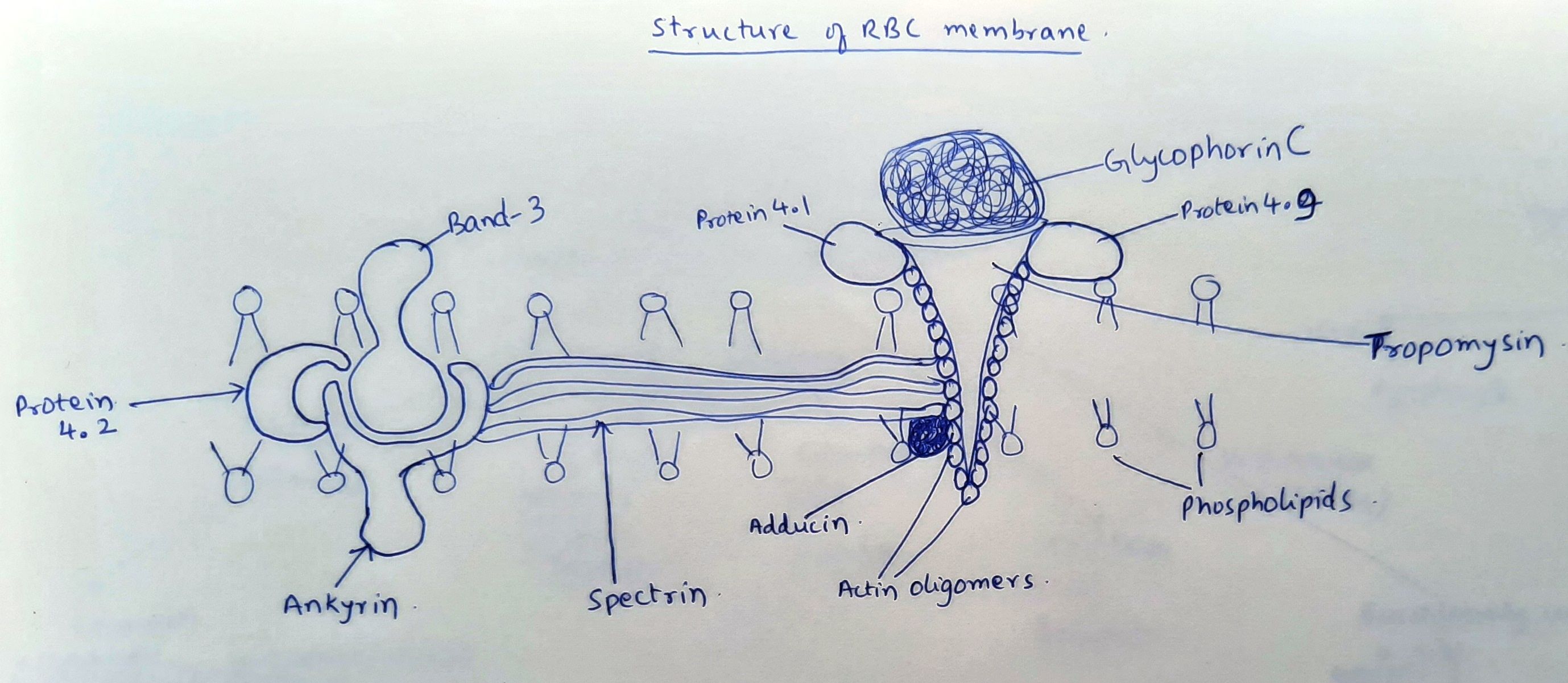
Figure1.2.1 - Structure of RBC membrane
Metabolism in RBC:
- Metabolism is limited in RBC because of lack of nucleus, mitochondria, and other sub-cellular organelles
- Important metabolic pathways include
- Embden Meyerhof pathway- Provides ATP for regulation of intracellular cation concentration (Na, K, Ca, Mg) via cation pumps.
- Hexose monophosphate shunt- Provides NADPH and glutathione to reduce cellular oxidants
- Rapoport Luebbering pathway- Forms 2,3 DPG which facilitates oxygen release to the tissues
- Methemoglobin reductase- Protects hemoglobin from oxidation via NADH and methemoglobin reductase
Structure and function of hemoglobin:
- Hemoglobin is a highly specialized intracellular protein, which is responsible for transport of oxygen and carbon dioxide.
- Each gram of Hb carries 1.34ml of oxygen.
- It occupies 33% of volume of RBCs.
- It accounts for 90% of dry weight of RBC
- Each RBC contains 27-32pg of Hb
- 65% of total Hb is produced before extrusion of nucleus. Remaining 35% is synthesized by the residual RNA and mitochondria within reticulocyte.
- Total amount of Hb in body- 750gm.
- 6.25gm of Hb is produced every day.
- 2X1011 RBCs are produced every day
- Structure of Hb consists of Tetramer with 2 pairs of globin chains and a heme molecule.
- Globin chains are synthesized in the polyribosomes in the cytoplasm.
- 2 alpha globins are common for all types of hemoglobins
- Non alpha globin chains in various hemoglobins include
- HbA- Beta
- HbA2- Delta
- HbF- Gamma
- Alpha chain gene is located on chromosome 16.
- Non alpha chain gene is located on chromosome 11.
- Switch over from gamma chain to beta chain synthesis occurs after the birth. The exact mechanism of this switchover is not understood.
- After 34-36 weeks of gestation, HbA rises, whereas HbF decreases.
- After birth, HbF level falls by 3% per week. By 6 months HbF level is <3% of total Hb.
- Normal HbA2 levels are achieved by 4 months of age.
- 75% of alpha and beta chains have helical arrangement. 8 helical areas (A-H) occur within the beta chain. Nomenclature includes number of helix and site of residue. For example, F8 means 8th residue in F helix, EF3 means 3rd residue of segment connecting E and F helices.
- Heme consists of tetra pyrrole (porphyrin) ring with ferrous iron located in the center of the ring.
- Steps in synthesis of heme are mentioned in “porphyria” chapter.
Oxygen disassociation curve:
- It is graphical representation of hemoglobin affinity for oxygen which determines the proportion of oxygen released to the tissues or loaded on to the RBC at a given oxygen pressure (PaO2)
- Saturation of Hb (%) is drawn on Y-axis while pO2 (mmHg) is drawn on X-axis.
- This curve is sigmoid shaped, as Hb is an allosteric protein.
- Because of this sigmoid shape, there is release of large quantities of oxygen with small changes in PaO2.
- At PaO2 of 50%, 50% of Hb is saturated.
- Shift of curve to right side indicates decreased O2 affinity. This occurs at tissue level and in conditions where CO2 levels are high, pH is low (Bohr effect), temperature is high and 2-3DPG level is high.
- Shift of curve to left side indicates decreased O2 affinity. This occurs at lung level.
Destruction of RBCs:
- Average life span of RBCs is 120 days.
- In aged RBCs, there is
- Decreased deformability
- Increased osmotic fragility
- Increased glycosylated hemoglobin
- Decreased lipid content in membrane
- Decreased G6PD and other enzymes
- Decreased ATP
- Aged RBCs expose phosphatidyl serine on the outer leaflet of cell membrane. Macrophages within spleen and liver recognize this and engulf such RBCs. This process is known as erythroptosis.
- Within the cytoplasm of macrophage, Hb is broken down into heme and globin. Globin becomes part of plasma proteins and joins general amino acid pool.
- Heme is broken down to biliverdin, carbon monoxide and iron. Iron returns to bone marrow. Carbon monoxide is exhaled through lungs.
- Biliverdin is converted to bilirubin and transported to liver after binding to albumin.
- In liver it is conjugated to form bilirubin diglucuronide, and then excreted in bile.
- Part of this returns to liver through enterohepatic circulation and part of it is converted to urobilinogen and stercobilinogen. Urobilinogen is excreted in both urine and stool, while stercobilinogen is excreted in stool.
- If there is intravascular lysis of RBCs, released free Hb gets bound to haptoglobin and is carried to liver, to form bilirubin. If there is excess of free Hb, it forms methemoglobin and later forms hemopexin. In case of massive intravascular hemolysis, hemoglobin is excreted as it is in urine (hemoglobinuria) or as hemosiderin (hemosiderinuria) which is formed after tubular reabsorption.
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.