howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Splenic B Cell lymphoma and leukemias (HCL, SMZL, SDRPL, SBLPN)
Updated on 20.02.2025
This category includes following disorders:
- Hairy Cell leukemia (HCL)
- Splenic Marginal Zone Lymphoma (SMZL)
- Splenic diffuse red pulp small B cell lymphoma (SDRPL)
- Splenic B cell lymphoma/ leukemia with prominent nucleoli (SBLPN)
Hairy Cell leukemia
Introduction:
- It is a neoplasm of small B-lymphoid cells with oval nuclei & abundant cytoplasm with hairy projections, diffusely infiltrating bone marrow & splenic red pulp, strongly expressing CD103, CD22 & CD11c and characterized by presence of BRAF V600E mutation.
Epidemiology:
- 2% of lymphoid leukemias
- Median age – 60 years
- M : F – 5 : 1
Etiology: Exact etiology is not known
- Radiation
- Organic solvents
- BRAF-V600E- mutation is seen in all cases of HCL
Pathogenesis:
1.
BRAF-V600E mutation
↓
Constitutive activation of the kinase BRAF
↓
Downstream signaling through MEK/ERK pathway
↓
Phosphorylation of several targets in the nucleus and cytoplasm
- Upregulation of General RAF-MEK-ERK Pathway Targets: MAFF, CCND1, ETV5, DUSP6, EGF1, SPRY2
- Upregulation of Proteins Characteristic of HCL: CD25 (IL2RA) and TRAP (ACP5)
- Induction of the HCL-Specific Gene Expression Signature: ACTB, CCND1, THBS1, AIF1, SPRY2, and others
- Induction of hairy morphology and inhibition of apoptosis
2.
Altered expression of chemokine and adhesion receptors + Over production of TNF, IL6 and BCL2
↓
Malignant transformation of mature memory B cell
↓
Homing into BM via constitutively activated integrin receptors and over-expression of matrix metalloprotease inhibitors
↓
Release of fibroblast growth factor and transforming growth factor beta 1 from hairy cells
↓
Increased fibronectin production in BM
↓
Reticulin fibrosis
Clinical Features:
- Asymptomatic
- Massive splenomegaly- 60-70% cases
- Rarely hepatomegaly, lymphadenopathy
- Pancytopenia
- Anemia
- Neutropenia – Frequent infections
- Thrombocytopenia – Bleeding tendency
- Common infections:
- Bacteria: M. avium intracellularae, Staphylococcus, Strep. pneumoniae, E. Coli, Pseudomonas aerogenosa.
- Others: CMV, PCP, Aspergillus, histoplasma, cryptococcus. Listeria, Toxoplasma
- Rare manifestations:
- Cutaneous vasculitis
- Leukocytoclastic angiitis
- Erythema nodosum
- Pulmonary infiltrates
- Polyarthritis
- Raynaud's phenomenon
- Osteolytic painful bony lesions (commonly involving proximal femur)
Investigations:
- Hemogram:
- Pancytopenia.
- Monocytopenia is a constant feature
- Hairy cells- Small to medium sized cells (usually twice the size of mature lymphocyte). Nucleus is oval / indented with homogeneous, spongy ground glass chromatin. Nucleoli are typically absent. Cytoplasm is abundant and pale blue. Circumferential hairy projections are present (Villous outlines). Discrete vacuoles are present.
- Bone marrow aspiration and biopsy
- Aspiration, as a rule, unsuccessful
- Interstitial or patchy involvement by tumour cells is noted
- Some preservation of fat and hematopoietic cells is present
- Leukemic cells- Widely spaced lymphoid cells creating a “honey comb” appearance
- Nucleus – Bean / round/ oval shaped
- Cytoplasm – Abundant with prominent cell borders (Fried egg appearance)
- Increased reticulin fibres (may result in a dry tap)
- Sometimes bone marrow can be hypoplastic due to cytokines produced by hairy cells
- Cytochemistry
- Tartrate resistant acid phosphates (TRAP)- Positive (Isoenzyme 5 acid phosphatase present in hairy cell cytoplasm resists decolorization by tartrate)
- Electron microscopy:
- Rod shaped inclusions that represent ribosome lamellar complexes are seen.
- Circumferential cytoplasmic projections
- Immunophenotype:
- Monocytic gate/ sometimes lymphocytic gate
- Positive – SIg M, B cell associated antigens (CD19, CD20, CD22, CD79a, CD200), CD11C, CD25, FMC7, CD103, CD123, Cyclin D, T bet (TBX-21)
- Negative - CD5, CD10, CD23, CD79b, CD21, CD27, CD38
- Tissue section- Positive for CD20, DBA 44, TRAP, CD72, Annexin A1 (Most specific), BRAF mutant protein, Cyclin D1, SOX11
- Cytogenetics
- No specific cytogenetic abnormality (Chromosome 5 is involved in 50% cases)
- Molecular studies
- Rearrangement of Ig light and heavy chains
- Over expression of Cyclin D1
- Molecular analysis for IGHV-34 gene rearrangements: 10% of HCL with typical immunophenotyping features have this arrangement. They lack BRAF mutation and behave like HCL variant. They have poor prognosis
- IHC or molecular analysis (NGS) for BRAF V600E mutation- If classical immunophenotyping features are not seen
Criteria for diagnosis:
Essential:
- Characteristic morphology in blood or bone marrow
- Strong positivity for CD20 and Annexin-1 by immunohistochemistry or coexpression of CD20/CD11c/CD103/CD25 by flow cytometry and/or immunohistochemistry.
Desirable:
- Clonal BRAF p.V600E (NP_004324.2) mutation.
- Useful adjuncts are the expression of CD123, bright CD22, bright CD200, bright surface immunoglobulins, cyclin-D1, and TBX21/T-Bet.
Prognosis:
- Without treatment median survival is 5-10 years
- With therapy- 4-year survival is up to 96%
- Remission rate with purine analogues is more than 90% and average duration of remission is 15 years.
- Modern therapy cannot cure this disease but can prolong the survival to near normal lifespan.
- Poor prognostic factors:
- Heavy BM involvement
- Massive splenomegaly
- Abdominal lymphadenopathy
- High beta 2 microglobulin
- Increased LDH
- CD38 expression
- Mutated IGHV
- Poor response to purine analogue
Differential Diagnosis:
- Splenic lymphoma with villous lymphocytes
- Marginal zone B-cell lymphoma
- Monocytoid B-cell lymphoma
- Small lymphocytic lymphoma
- Primary splenic chronic lymphocytic leukemia
- Lymphoplasmacytoid lymphoma
- Chronic lymphocytic leukemia
- Mantle zone (intermediate) lymphoma
- Myeloproliferative disorders
- Malignant histiocytosis
- Primary lymphoma of spleen
- Chronic prolymphocytic leukemia
- Large granular lymphocytic leukemia
- Systemic mast cell disease
- Hairy B-cell lymphoproliferative disorder
Indications for Treatment:
- Systemic symptoms such as weight loss (>10% within 6 months), excessive fatigue
- Splenic discomfort
- Recurrent infections
- Hemoglobin <10g/dL
- Platelets <1,00,000/cmm
- ANC- <1000/cmm
- Symptomatic organomegaly
- Progressive lymphocytosis or lymphadenopathy
- Bone involvement
Pretreatment Work-up:
- History
- B-Symptoms
- Examination
- LN:
- Spleen:
- WHO P. S.
- BSA
- Hemoglobin
- TLC, DLC
- Platelet count
- BMA and Bx
- IHC/Flow cytometry
- IHC for mutant BRAF protein (VE1)
- PCR/ NGS for BRAF V600E mutation
- CT (CAP)
- Stage
- LFT: Bili- T/D SGPT: SGOT: Albumin: Globulin:
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid:
- LDH
- HIV:
- HBsAg:
- HCV:
- UPT
- Cytogenetics
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
Treatment Plan:
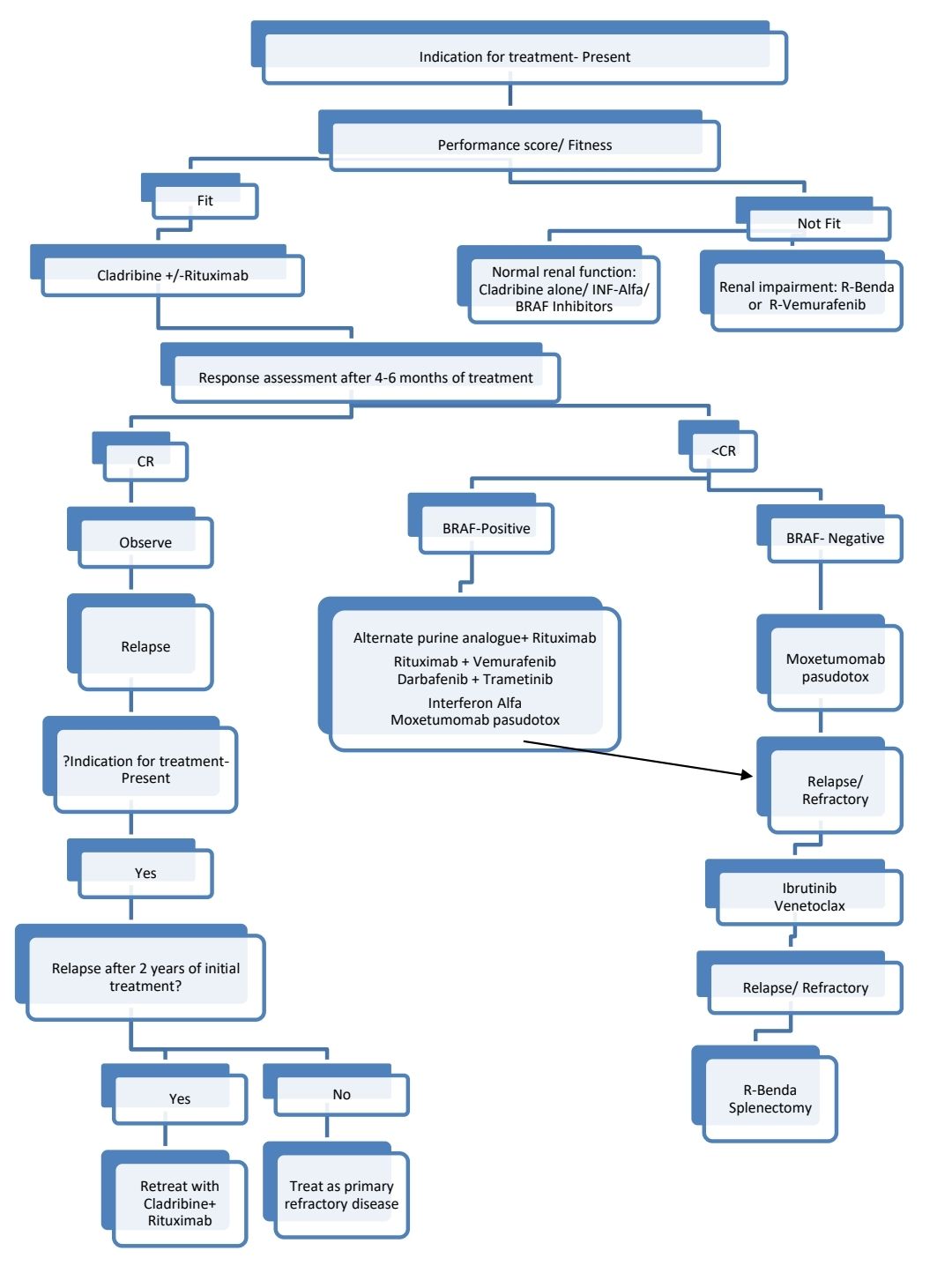
Response Criteria (Assessment must be done 4-6 months after Cladribine therapy):
- Complete response:
- Near normalization of peripheral blood counts (Hb>11gm/dL, PL >1lac/cmm, ANC >1500/cmm)
- Regression of splenomegaly on physical examination
- Absence of morphological evidence of HCL on both PS and BMA.
- Partial remission:
- Normalization of cytopenias along with minimum 50% improvement in both organomegaly and BM infiltration with no circulating hairy cells
About Each Modality of Treatment:
- Cladribine (+/- Rituximab)
- 2 dosing regimens:
- 0.14mg/kg/day- IV- over 2 hrs-OD- for 5 days
- 0.1mg/kg/ Day- as continuous IV infusion for 7days
- 90% have chronic remission
- 26% relapse at median of 29 months
- Treat active life threatening or chronic infections prior to starting chemotherapy.
- G-CSF may be used after cladribine to decrease the period of neutropenia
- If Rituximab is being added, start it 1-2 months after completion of cladribine. Give Weekly for 8 weeks.
- 2 dosing regimens:
- Interferon alfa 2b
- Given for 1 year
- Rarely used
- Useful in patients with active infections
- 5% CR and 70% PR
- Pentostatin
- Acts by inhibiting adenosine deaminase
- 4mg/m2 every 2 weeks until maximal response (usually 8-10 injections) then 2 extra injections
- Given for 3-6 months
- Produces neurotoxicity and skin rash
- Avoid if creatinine clearance is <60 ml/min, 50% dose if 40-60 ml/min
- Splenectomy
- Improves quality of life and prolongs survival
- 75% response rate
- Indications
- Uncontrolled infections: As post-splenectomy rise in ANC can control infections effectively
- Increase in size >10cm below costal line with very less bone marrow involvement
- Alternate treatment should be considered 6 months after splenectomy
- Rituximab
- Useful in relapsed cases
- 375mg/m2 IV weekly with purine analogues for 8 weeks
- Moxetumumab pasudotox
- Recombinant immunotoxin directed against CD22 and linked to a truncated Pseudomonas exotoxin.
- Response rate- 86% in heavily pretreated patients.
- Dose: 40micrograms/kg-IV- Given on days 1, 3, and 5 of each 28 days cycle for up to 6 cycles (Stop if CR is achieved)
- Good pre-hydration should be given.
- Side effects- Hemolytic uremic syndrome, capillary leak syndrome.
- Moxe retreatment is also effective
- BRAF-MEK-ERK pathway inhibitors
- Vemurafenib-
- 960mg- BD- daily for 16-18 weeks
- Response rate- 96%
- Side effects- Skin rash, palmar/plantar fibrosis, skin warts, skin tumors, arthritis
- When Rituximab is added, it is given once in 2 weeks for 8 doses
- Dabrafenib
- Dose: 150mg- BD for 8 weeks (additional 4 weeks for patients who do not achieve CR)
- ORR: 80%
- Trametinib
- Dose: 2mg- OD
- Usually combined with Dabrafenib
- Vemurafenib-
- Ibrutinib:
- Dose: 420mg- OD
- ORR- 24% (In heavily pretreated patients)
- Venetoclax
- Each cycle of 28 days for up to 12 cycles.
- May be added with Rituximab
Supportive Care:
- Herpes virus prophylaxis is must during treatment and 3 months thereafter (until CD4 count >200/cmm)
- PCP prophylaxis for same duration
- Give only irradiated blood products lifelong if patients are treated with purine analogues
- Radiation for lytic bone lesion (1500-3000 rads)
Monitoring After Treatment/ Follow-up:
- Long term survivors are at increased risk of HD, NHL and thyroid malignancies
Special Situations:
- Pregnancy:
- Avoid chemotherapy as far as possible. If inevitable, INF is a reasonable choice.
Related Disorders:
- Blastic variant of HCL
- Massive splenomegaly
- Peripheral adenopathy and cytopenias
- TRAP- Positive, MPO- Negative
Splenic Marginal Zone Lymphoma
Introduction:
- It is a B-cell neoplasm comprising of small lymphocytes which surround and replace the splenic white pulp germinal centers, efface the follicle mantle and merge with a peripheral zone of larger cells including scattered transformed blasts; both small and large cell infiltrate the red pulp.
- It is also called as splenic lymphoma with villous lymphocytes.
Epidemiology:
- 2% of lymphoid neoplasms
- Median age- 69 years
- M:F- 1:1
Etiology:
- HCV infection
Pathogenesis:
- NOTCH pathway and other marginal zone differentiation-associated genes are frequently mutated
- Mutations involving the KLF2 transcription factor, leading to activation of NF-kB signaling
Clinical Features:
- Splenomegaly
- B Symptoms
- Autoimmune manifestations
- Acquired angioedema due to C1-esterase inhibitor deficiency
- Cold agglutinin disease and warm antibody autoimmune haemolytic anaemia
- Immune thrombocytopenia
- Mixed cryoglobulinemia
Investigations:
- Peripheral smear- Small lymphoma cells which have a characteristic short polar villi (confined to one pole) & irregular membrane outline. Nucleolus is seen in half of cases
- Bone marrow aspiration:
- Similar cells infiltrate in nodular/ interstitial pattern
- Intrasinusoidal lymphocytic infiltration is characteristic
- Immunophenotype
- Positive - Surface IgM& IgD, CD20, CD79a, CD11c , PAX5, FMC7, CD27, CD38 (dim)
- Negative - CD5, CD10, CD23, Annexin A1, Nuclear Cyclin D1, CD103, CD10, BCL6, SOX11, LEF1
- Variable- CD23, CD43, DBA44, CD25
- Ki 67 staining- Targetoid pattern with increased growth fraction in both germinal and marginal zones
- Molecular studies
- Rearrangement of Ig heavy and light chain genes.
- Loss of Chr. 7q 31-32 which leads to inactivation of p53
- Trisomies of chromosome 3 and 18
- Dysregulation of CDK gene on 7q21
- Mutations in KLF2 and NOTCH2 genes
- S. Protein electrophoresis- Some may have small monoclonal M protein
Criteria for Diagnosis:
Essential:
- Small B-cell lymphoma involving bone marrow and/or peripheral blood composed of small lymphoid cells with villous processes
- Neoplastic cells express pan-B cell markers, IgM and IgD and are negative for BCL6, annexin A1, CD103, cyclin D1, SOX11 and LEF1
- Other splenic and nodal B-cell lymphomas should be excluded.
- Clinical or imaging studies that show splenomegaly.
Desirable:
- Neoplastic cells negative for CD5 and CD10
Prognosis:
- It has an indolent course, with median survival- >10 years
- Prognostic markers:
- Hemoglobin: <12gm/dL
- Elevated LDH
- Albumin- <3.5g/dL
Number of markers present | Risk | OS at 5 years |
0 | Low | 88% |
1 | Intermediate | 73% |
2 or 3 | High | 50% |
Differential Diagnosis:
- CLL
- HCL
- Mantle cell lymphoma
- Follicular lymphoma
- Lymphoplasmacytic lymphoma
Indications for Treatment (Even at relapse):
- Symptomatic patient (weight loss >10% in <6 months, fever, drenching sweats)
- Progressive/ symptomatic spenomegaly
- Bulky lymphadenopathy
- Autoimmune phenomenon
- Progressive cytopenia
- Compromised vital organs
- Hepatitis C positive with splenomegaly
Pretreatment Work-up:
- History
- B-Symptoms
- Examination
- LN:
- Spleen:
- WHO P. S.
- BSA
- IHC/Flow cytometry
- BMA and Bx
- CT (CAP) Or Whole body PET if transformation is suspected
- Stage
- Hemoglobin
- TLC, DLC
- Platelet count
- LFT: Bili- T/D SGPT: SGOT: Albumin: Globulin:
- SPEP/ IFEP
- Creatinine
- Electrolytes: Na: K: Ca: Mg: PO4:
- Uric acid:
- LDH
- HIV:
- HBsAg:
- HCV:
- UPT
- Sperm banking
- Cytogenetics
- ECHO(If anthracyclines planned)- LVEF- %
- Chemotherapy consent after informing about disease, prognosis, cost of therapy, side effects, hygiene, food and contraception
- Fertility preservation
- PICC line insertion and Chest X ray after line insertion
- Tumor board meeting and decision
- Attach supportive care drug sheet
- Inform primary care physician
Treatment Plan:
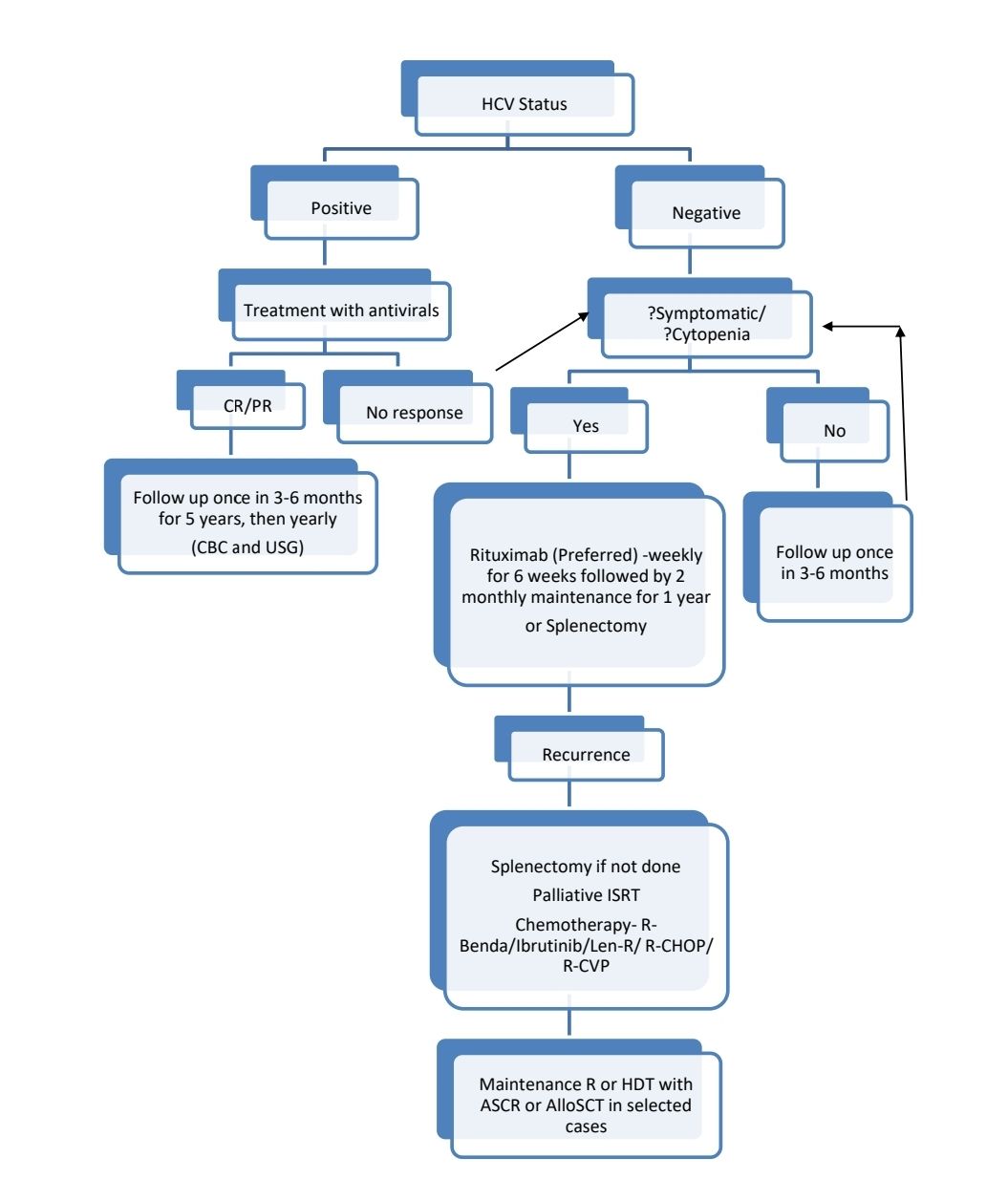
Response Criteria:
- Complete response:
- Recovery of counts
- Normal bone marrow with no excess lymphocytes
- Regression of splenomegaly
About each modality of treatment:
- Splenectomy:
- Useful in selected patients Ex: rituximab, BTK inhibitor refractory disease
- Not useful if there is disseminated nodal involvement or BM involvement.
- Laporoscopic approach is better
- Vaccinations must be done 2 weeks prior to surgery
- VTE prophylaxis should be given
- Chemotherapy:
- BR is preferred over single agent Rituximab in patients with CD5 expression
- BTK inhibitors:
- May be combined with Venetoclax or Rituximab
- CAR-T cell therapy
- Axicabtagene ciloleucel: Useful in patients who have failed multiple lines of therapy
Monitoring After Treatment/ Follow-up:
- Once in 3-6 months for 5 years, then once a year
Splenic diffuse red pulp small B cell lymphoma (SDRPL)
- Diagnosis is made after excluding CLL, HCL, LPL, PLL, vHCL
- Rare (<1% of all NHL)
- Present with massive splenomegaly
- Peripheral smear shows small to medium sized villous lymphocytes (Similar to seen with SMZL) with leucopenia and thrombocytopenia
- BM shows intrasinusoidal infiltration
- Spleen- Diffuse pattern with red pulp involvement. Both cord and sinusoidal infiltration are present.
- TRAP- Negative
- Immunophenotyping
- Positive- CD20, CD19, CD79a, DBA.44, IgG, Cyclin D3
- Negative- IgD, Annexin 1, CD25, CD5, CD103, CD123, CD11c, CD10, CD23, CD43, cyclin D1, CD21, CD38
- SPE may show monoclonal band
- Molecular studies
- Hypermutation in IgHV genes
- t(9:14) involving PAX5 and IgH @genes
- TP53 mutations
- Criteria for diagnosis
- Essential:
- Diffuse infiltration of the spleen by monomorphic small B cells, accompanied by atrophic white pulp
- Peripheral blood with circulating small cells with abundant cytoplasm, broad-based and unevenly distributed cytoplasmic villous projections are well-visible, and inconspicuous nucleolus
- Immunophenotype compatible with SDRPL
- Desirable:
- Absence of BRAF p.V600E (NP_004324.2) mutation
- Absence of lymphadenopathy other than splenic hilar lymph node involvement.
- Essential:
- Prognosis: Indolent course
- Treatment:
- Treat only if indication for treatment is present
- First line: Splenectomy
- Ineligible for splenectomry or R/R: Rituximab monotherapy, R+Cladribine, R+Bendamustine
Splenic B cell lymphoma/ leukemia with prominent nucleoli (SBLPN)
- Includes cases which were previously called hairy cell leukemia- variant and CD5 Negative B- Prolymphocytic leukemia.
- It is considered hydrid between hairy cell leukemia, B-prolymphocytic leukemia and marginal zone lymphoma
- It is associated with high WBC count (50-80 x 109/L) and splenomegaly
- Morphologically compared to HCL, cells are medium to large, have distinct, large, single nucleolus and round nucleus with irregular nuclear contours. Cytoplasm is abundant, basophilic and lacks hairy projections.
- BM biopsy- Infiltrates are subtle and vary inconspicuous intra-sinusoidal infiltration
- TRAP- Negative
- BRAF V600E mutation- Negative
- MAP2K1 mutations- 50% of cases
- Cytogenetics: Complex karyotypes involving 14q32 as well as 8q24, deletion 17p, and trisomy 12
- Immunophenotyping
- Positive- CD103, CD11C, FMC7, surface immunoglobulin (more frequently IgG), pan-B cell antigens (CD19, CD20 and CD22, DBA-44)
- Negative- CD25, CD123, Annexin 1, HC2, TRAP
- Criteria for diagnosis:
- Essential
- Circulating medium-sized lymphoid cells with prominent nucleoli or convoluted nuclei and absence of hairy projections
- Presence of B-cell antigens CD19, CD20, CD79a, or PAX5;
- Absence of characteristic phenotype of HCL, including expression of CD25, annexin A1, cyclin D1, and TRAP.
- Desirable
- Diffuse involvement of the splenic red pulp with atrophic white pulp, but most cases are diagnosed without a spleen specimen;
- Absence of BRAF mutation.
- Essential
- Prognosis: Median survival- 9 years
- Treatment
- Treatment to be given only if indication for treatment is present (same as HCL)
- They poorly respond to cladribine and pentostatin (Do not respond to IFN-alfa)
- Rituximab with Cladribine is the better option.
- Splenectomy- Good partial response in 2/3rd patients
- Options in relapse/ refractory situations: R-Bendamustine, Ibrutinib +/- Venetoclax
Immunophenotype by Immunohistochemistry
Marker | SDRPL | SMZL | SBLPN | HCL |
|---|---|---|---|---|
| Cyclin D1 | - | - | - | + |
| Cyclin D3 | - | - | - | + |
| Annexin A1 | - | - | - | + |
Immunophenotype by Flow Cytometry
Marker | SDRPL | SMZL | SBLPN | HCL |
|---|---|---|---|---|
| CD11c | Bright in ~67% of cases | Moderate in ~70% of cases | Bright in ~25% of cases | Bright in 100% of cases |
| CD180 | Strong in 100% of cases | Moderate in 93% of cases | Not described | Strong in 100% of cases |
| CD200 | Dim in 40% of cases | Moderate in 93% of cases | Not described | Strong in 100% of cases |
| CD103 | Positive in ~20% of cases | Negative | Positive in 65–100% of cases | Bright in 100% of cases |
| CD123 | Positive in 3% of cases | Negative | Positive in 9% of cases | Bright Positive in 100% |
Molecular Profile
Parameter | SDRPL | SMZL | SBLPN | HCL |
|---|---|---|---|---|
| IGHV1-2*04 gene usage | <5% | ~30% | Unknown | <1% |
| del(7q) | ~25% | ~40% | ~20% | ~20% |
| NOTCH2 mutations | <2% | 15–20% | 0% | 0% |
| KLF2 mutations | <2% | ~20% | 0% | 15% |
| CCND3 mutations | ~25% | 0% | Unknown | 0% |
| BCOR alterations | ~24% | <10% | Unknown | <10% |
| BRAF V.600E | <1% | <1% | <10% | ≥95% |
Figures:
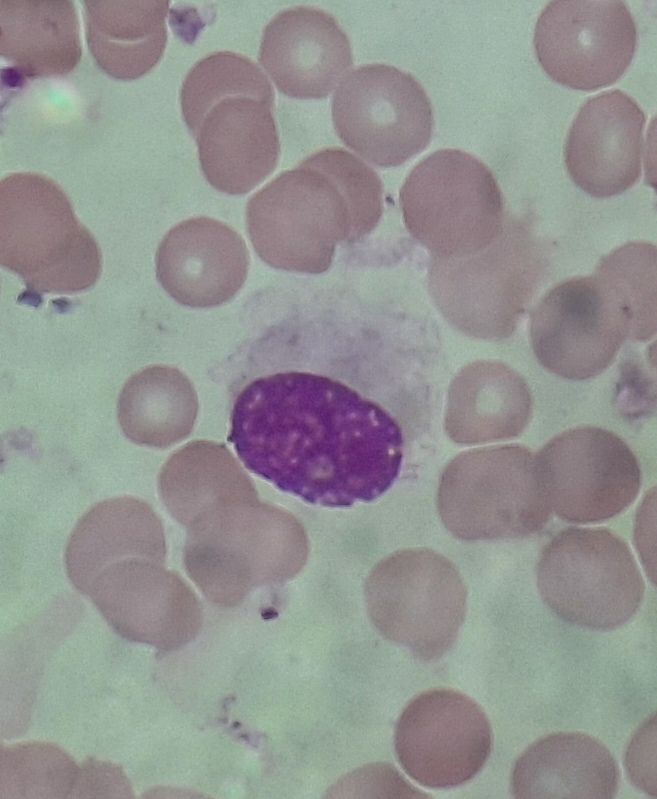
Figure 6.5.1- Hairy cell in peripheral smear
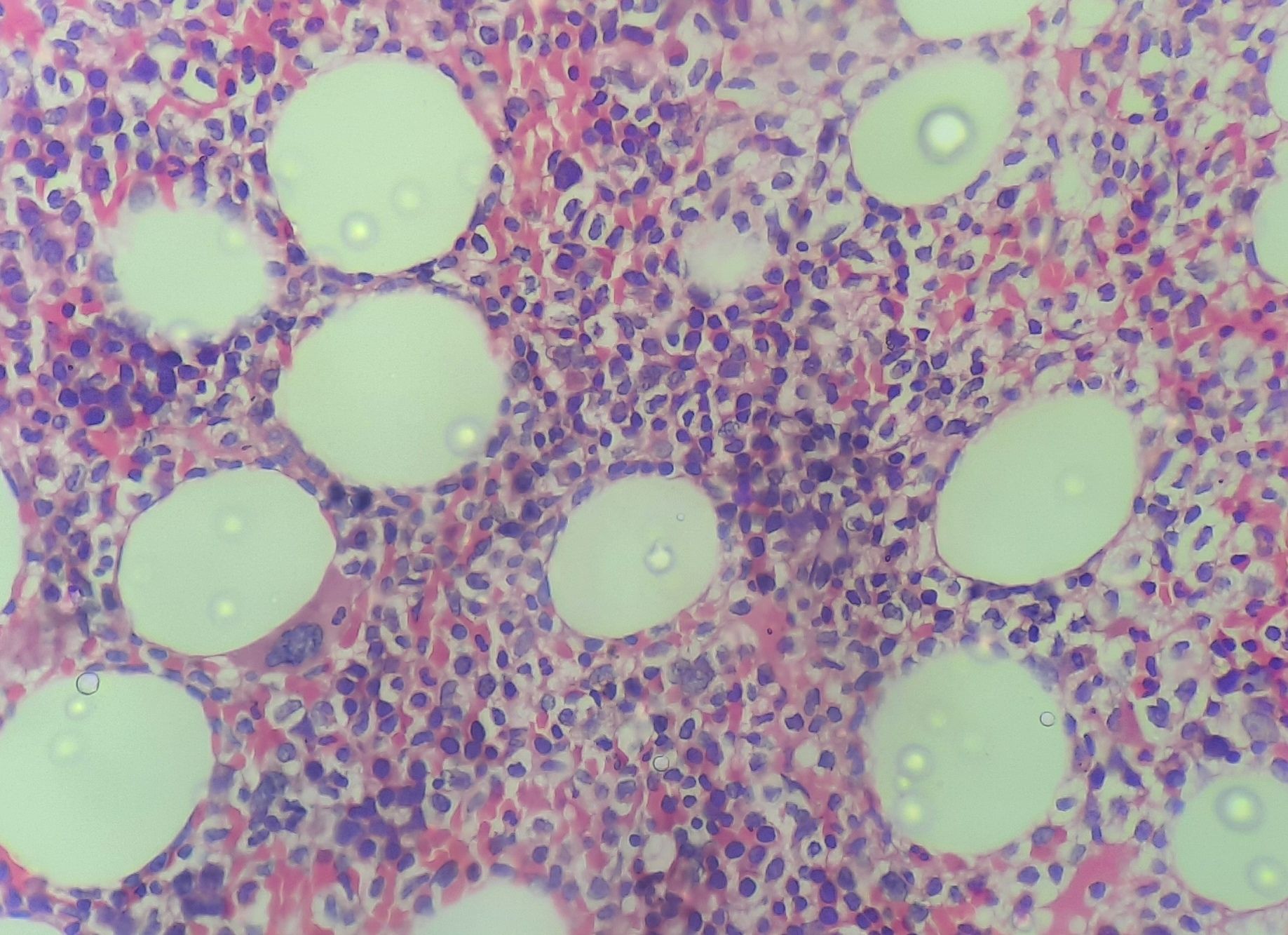
Figure 6.5.2- Hairy cell leukemia- Bone marrow biopsy
Recent advances:
Long-term outcomes in patients with relapsed or refractory hairy cell leukemia treated with vemurafenib monotherapy
Vemurafenib, an oral BRAF inhibitor, has demonstrated high response rates in relapsed/refractory (R/R) hairy cell leukemia (HCL). Present study reports results of 36 patients with R/R HCL treated with vemurafenib. The best overall response rate was 86%, including 33% complete response and 53% partial response. Overall survival was 82% at 4 years, with a significantly shorter OS in patients who relapsed within 1 year of initial treatment with vemurafenib. All adverse events in the retreatment cohort were grade 1/2 except for 1 case of a grade 3 rash and 1 grade 3 fever/pneumonia..
https://doi.org/10.1182/blood.2022016183
Venetoclax in Relapsed or Refractory Hairy-Cell Leukemia
In 6 patients with hairy-cell leukemia and progressive disease after vemurafenib plus rituximab, 5 had a response to venetoclax alone or with the addition of rituximab, including 3 complete responses.
https://doi.org/10.1056/NEJMc2216135
Dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600E mutation–positive hairy cell leukemia
Patients received dabrafenib 150 mg twice daily plus trametinib 2 mg once daily until disease progression, unacceptable toxicity, or death. ORR was 89.0% and 65.5% of patients had a complete response. The 24-month DOR was 97.7% with 24-month PFS and OS rates of 94.4% and 94.5%, respectively.
https://doi.org/10.1182/blood.2021013658
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.