howitreat.in
A user-friendly, frequently updated reference guide that aligns with international guidelines and protocols.
Thrombotic Thrombocytopenic Purpura
Introduction:
- It is a syndrome characterized by microangiopathic hemolytic anemia, thrombocytopenia, and elevation of LDH and formation of hyaline fibrin microthrombi.
- It may be associated with noninfectious fever, neurological disorder and renal abnormality.
- It occurs due to deficiency of ADMTS- 13 protein, which is a metalloprotease that cleaves and destroys von Willebrand factor multimers
- TTP has usually neurological involvement, whereas HUS has usually renal involvement.
Epidemiology:
- Incidence- 4 cases/ 1 million population
- M:F- 1:2
- Peak age- 30-50 years
Etiology:
- Immune- Auto antibodies against ADMTS-13
- Primary
- Secondary- SLE, Graves' disease, HIV, Drugs and pregnancy related TTP
- Congenital (Upshaw Schulman syndrome)
- Autosomal Recessive
- Mutation of ADMTS-13 gene on chromosome 9q34
(Plasma exchange is useful only in immune related TTP)
Pathogenesis:
Deficiency of protein ADMTS-13
↓
Persistence of unusually large VWF multimers
↓
Platelet aggregation and formation of plugs within small blood vessels
(Multimeric VWF that is released upon minor endothelial damage is not destroyed)
↓
- Ischemic organ damage
- Consumption of platelets leading to thrombocytopenia.
- Passing of RBCs through fibrin meshwork leading to microangiopathic hemolytic anemia.
- Person with relatively normal ADMTS-13 may have TTP and those with decreased levels may not have TTP. So ADMTS-13 deficiency is important predisposing factor, but not the sole cause for this syndrome
Other Suspected mechanisms
- Endothelial cell activation
- Increased P selectin
- Decreased prostacyclin
- Endothelial apoptosis
- Platelet activation/aggregation
- Platelet aggregating proteins other than VWF such as Cysteine proteases (calpains, cathepsins)
Clinical Features: (Symptoms develop over weeks)
- Anemia- Fatigue, pallor
- Jaundice
- Thrombocytopenia: Bleeding and petechiae, Epistaxis, gingival bleeding, menorrhagia
- Fever
- Arthralgia, myalgia
- Renal- Proteinuria, microhematuria, renal failure is rare
- Neurological abnormalities that wax and wane over minutes (Fluctuations in severity is due to repetitive formation and dissolution of thrombi)
Headache, confusion, aphasia, alteration of consciousness
↓
Hemiparesis, seizures, sensory/motor deficits
↓
Coma
- Abdominal pain and tenderness due to pancreatitis/ intestinal ischemia
- Cardiac- Chest pain, heart failure, hypotension
Investigations:
- Hemogram
- Hemoglobin concentration-Decreased (Median 8-10gm/dL)
- Hematocrit- Less than 20%
- RBC- Fragmented RBCs (schistocytes), helmet cells, triangle forms etc. 1-18% of total RBCs are fragmented RBCs.
- Many polychromatophilic cells are seen along with nucleated RBCs.
- WBC- Leucocytosis.
- Platelets – Thrombocytopenia (10-30x109/L)
- LDH-Elevated to more than 1000 units/Lit
- Bilirubin – Elevated (Predominantly indirect)
- S. Haptoglobin- Decreased
- Urine – Hemoglobinuria
- Coomb’s test – Negative
- Coagulation tests – Normal
- RFT- Usually normal
- HIV, HBsAg and HCV- Needed to rule out HIV associated TTP and as baseline prior to plasma exposure/ Rituximab
- ANA profile and APLA work up- To rule out secondary causes
- Pregnancy test- To rule out pregnancy associated TTP/ microangiopathies
- Troponin T and I- To know cardiac involvement
- Thyroid function tests- For Grave's disease associated TTP.
- Amylase and lipase- As rarely TTP may be associated with pancreatitis
- CT/MRI
- To determine CNS involvement
- It should interrupt the process of plasma exchange
- CT chest/abdomen/pelvis +/- tumor markers- To look for underlying malignancy
- Gingival biopsy
- Typical micro thrombi in arterioles, capillaries and venules with no inflammation in vessel wall.
- Arterioles are filled with hyaline material possibly consisting of fibrin and platelets, without inflammatory changes in vessel wall.
- Microaneurysms of arterioles are usually present.
- ADAMTS-13 levels –Reduced to <5%
- To be done on citrated plasma
- Should send pretreatment samples i.e. prior to plasma exchange.
- Other conditions with reduced levels of ADMTS -13 (<40% but >5%)
- Neonates
- Pregnancy
- Localized & metastatic cancer
- HELLP syndrome
- Liver cirrhosis
- Inflammatory states/ Sepsis
- DIC
- Post operative period
- uremia
- Autoimmune diseases
- Anti ADAMTS antibodies (IgG)
Criteria for Diagnosis: Diagnostic Pentad (All need not be present)
- Thrombocytopenia with normal coagulation parameters (PT, APTT, Fibrinogen)
- Microangiopathic hemolytic anemia (Schistocytes in PS with high LDH)
- Neurological changes
- Fever
- Renal dysfunction (Predominates in HUS)
Diagnosis is confirmed by demonstrating severe deficiency of ADAMTS13 (<10IU/dL).Treatment (PEX etc) must be started pending this report.
Prognosis:
- Mortality is 80-90% without plasma exchange therapy
- Mortality is10-20% with plasma exchange therapy (Most of these deaths occur within few days of admission)
- Average time for complete response- 9-16 days. (2-40 days)
- Relapse chance- 25-50%
Differential Diagnosis:
- Other causes of microangiopathic hemolytic anemias
Plasmic score:
- Used in patients with suspected TTP, to identify those patients who require urgent plasma exchange. This should not be used in patients who have already undergone plasma exchange.
- It is useful in identifying patients who require urgent ADMTS-13 assessment and subsequent plasma exchange.
- Patients in low-intermediate risk group (scores 0-5), do not show good response to plasma exchange.
- Factors taken into account (Each- 1 point)
- Platelet count- <30,000/cmm
- Evidence of hemolysis (High reticulocyte count, undetectable haptoglobin, Indirect bilirubin >2mg/dL)
- No active cancer
- No history of solid organ or stem cell transplant
- MCV- <90fL
- INR- <1.5
- Creatinine- <2mg/dL
Pretreatment Work-up:
- TTP must be considered as medical emergency.
- Plasma therapy must be initiated within 4-8 hours.
- Initial diagnosis must be made based on history, examination and routine investigations including peripheral smear examination. Treatment should be started without waiting for ADAMTS-13 protein levels.
- If there is delay in starting plasma exchange, give large volume FFP infusion (30ml/Kg). Watch for fluid overload.
- Even coma is not a contraindication for treatment, as full neurological recovery is a rule in patient responding to treatment
- Avoid platelet transfusions, as it increases the risk of mortality.
Work up must include:
- History
- OCPs/CSA
- Examination
- LN:
- Spleen:
- Hemoglobin
- TLC, DLC
- Platelet count
- Peripheral Smear
- Schistocytes:
- Reticulocyte count
- Haptoglobulin
- DCT
- LDH
- Clotting screen: PT: APTT: Fibrinogen:
- Blood group and antibody screen
- BMA and Bx(If other diagnosis suspected)
- CT/MRI Brain(After PEX)
- LFT-Bili- T/D SGPT: SGOT:Albumin: Globulin:
- Amylase
- RFT: Creatinine: Urea:
- TFT- T3: T4: TSH:
- ANA- by IF
- Rheumatoid factor
- Lupus anticoagulants
- Stool culture (for E.Coli) If diarrhea +
- CT (C/A/P)+/- Tumor markers(If malignancy suspected)
- Electrolytes: Na: K: Ca:Mg: PO4:
- Uric acid:
- Troponin I
- ECG
- BNP
- HIV:
- HBsAg:
- HCV:
- Urine routine
- UPT(Women of child bearing age)
- ADAM TS 13 assay
- ADAMTS 13 antibody assay
- ECG
- ECHO
- C3
- C4
- Urine for hemosiderin
- Plasmic score
Treatment Plan:
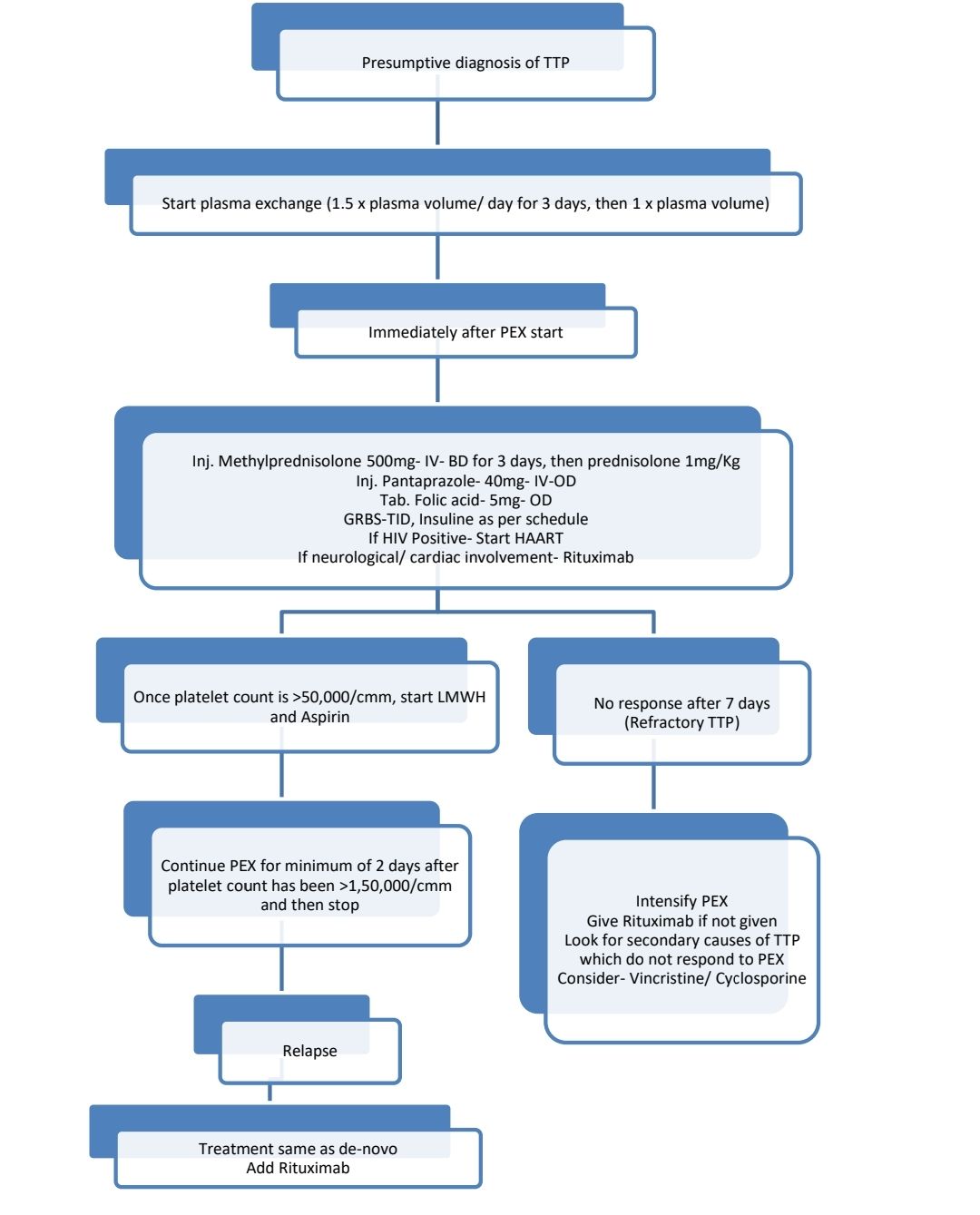
Daily investigations: CBC, LDH, bilirubin, Creatinine
About Each Modality of Treatment:
- Plasmapheresis-
- Removal of antibody & replacement of metalloproteinase by fresh frozen plasma.
- 1.5 plasma volume (3-6liters) exchange daily for 3 days and then 1 plasma volume
- Plasma volume (mL) = body weight (kg) × 70 (ml/kg) × (1 − hematocrit value [%]/100).
- If it is difficult to calculate plasma volume, use 50-75ml FFP/Kg
- Each bag of FFP contains approximately 200ml of plasma.
- Cryosupernatant is preferred as it has low VWF concentration
- Use solvent/ detergent treated plasma if available
- In case of Jehovah’s witness patients, use albumin instead of plasma
- If there is delay in plasma exchange, transfuse FFP- 15ml/kg.
- Monitor the treatment by
- Blood film for fragmented RBCs
- Platelet count
- LDH
- Steroids-
- Methylprednisolone 500mg IV- BD for 3 days followed by Prednisolone-1-2-mg/kg/day- for 2 weeks, then taper to 0.5mg/Kg over 2 weeks, then decrease 2.5-5mg every week, taking note ofplatelet counts.
- Give immediately after PEX
- PPI for ulcer prophylaxis
- T. Folic acid- 5mg-OD
- Rituximab
- Shortens the time to remission and reduces relapse rates
- Should not be given in pregnancy
- Use with caution in HIV, HBV, and HCV patients
- 375mg/m2- once a week for 4 weeks
- Give immediately after PEX
- Indications
- Neurological/ cardiac involvement
- Refractory TTP (No response after 7 days of plasma exchange and steroids)
- Relapsed TTP
- Withhold PEX for at least 4 hrs after Rituximab infusion
- Use obinutuzumab if patient develops anaphylaxis to Rituximab
- Antibiotics- useful as infection usually precipitate episodes
- Aspirin-
- Dose- 75mg- OD
- Start once platelet count is >50,000/cmm
- Clopidogrel and Ticlopidine are not used as they promote TTP
- LMWH in prophylactic doses
- Start once platelet count is >50,000/cmm
- Stop once patient is fully ambulant and off PEX for >10 days
- Hepatitis B vaccination once platelet count >50,000/cmm
- Vincristine
- May be useful in resistant cases
- Dose: 2mg- IV- on days 1, 4 and 7
- Cyclosporine
- Useful in maintaining remission in chronically relapsing disease
- Dose- 2-3mg/kg/day- in two divided doses
Supportive Care:
- PRBC transfusions to keep hemoglobin level >7gm/dL
- HBV vaccination once platelet count is >50,000/cmm
- Platelet transfusions are generally contraindicated, except in cases of life-threatening bleeding
Other Treatment Options:
- Recombinant ADMTS-13 therapy- Not yet available
- Anti-vWF therapy targeting GpIb alpha binding site
- Aptamer- Humanised monoclonal antibody
- Caplacizumab-
- Anti-VWF nanobody (Monoclonal, bivalent humanized Immunoglobulin fragment).
- Binds to A1 region of VWF, preventing platelet binding to the GpIb-IX-V receptor. Hence decreases thrombosis.
- Dose- 10mg (If <40kg- 5mg)- IV- Before PEX for 1st dose, then 10mg- SC-OD after PEX. After stopping PEX continue Caplacizumab for next 30 days. If ADAMTS levels are <20IU/dL, it may be continued beyond 30 days.
- Shortens the time to platelet response
- Bivalent single domain antibody which
- Used along with PEX and steroids
Follow up:
- Risk of relapse is 30-50%. Hence lifelong follow up is necessary.
- Neurocognitive assessment and psychology support must be offered to patients developing anxiety/ depression.
- Patients may be monitored with regular ADAMTS 13 levels. If levels are <20%, Rituximab (Standard dose/ low dose) may be administered prophylactically.
- Frequency of monitoring ADAMTS 13 levels may be reduced once the levels stabilize.
Related Disorders:
- Congenital TTP (Upshaw Schulman syndrome)
- More than 150 mutations reported so far
- Autosomal recessive inheritance
- Neonatal onset of hemolytic anemia and thrombocytopenia. But can present even up to age of 50-60 years.
- Several mild phenotypes are also there.
- Accounts for 2-10% of all TTP cases.
- ADAMTS-13 activity is <5%, in absence of antibodies.
- Molecular diagnosis may be used to confirm the diagnosis
- Persistent low ADAMTS-13 levels, but patients become symptomatic when exposed to precipitating factors such as infection, vaccination, excess alcohol and pregnancy.
- Responds to fresh frozen plasma infusion (Exchange is not required)
- Dose: 10-15ml/kg
- Frequency depends on patient's phenotype. Generally administered once in 3 weeks
- Consider testing siblings and first-degree relatives
- Pregnancy related TTP
- 1st trimester: Pregnancy can be continued along with routine treatment including plasma exchange
- Last trimester: Delivery is definitive treatment for all pregnancy related microangiopathies. However, as for any other case, plasma exchange and other treatments have to be given.
- During subsequent pregnancies, monitor ADAMTS-13 levels once in 3 months.
- HIV related TTP
- Treatment is same as routine treatment including plasma exchange and steroids.
- Continue HAART/ Start if not started
- As CD4 count recovers, there is normalization of ADAMTS-13 activity
- Rituximab can be given if CD4 count is >50/cmm
- Drug induced TTP
- Stop offending drug
- PEX helps in some cases
- BMT associated TTP
- Occurs because of endothelial damage caused by high dose chemotherapy/ radiation, immunosuppressive drugs, GVHD or infections
- Management is difficult, as stopping CSA and starting alternate immunosuppression can aggravate GVHD
- No benefit of plasma exchange. In fact it increases the mortality.
- Defibrotide may be useful
- Malignancy associated TTP
- No benefit of plasma exchange
- Underlying malignancy must be treated
- Hemolytic Uremic Syndrome:
- Important to differentiate from TTP as prognosis and management are different.
- Primary difference between TTP and HUS is presence of oliguric/ anuric renal impairment/ failure.
- No ADMTS-13 deficiency
- Caused by shiga toxin producing bacteria such as E. Coli 0157:H7, which is present in meat of infected cattle
- Pathogenesis
- After binding to Gb3, holotoxin is internalized and transported to endoplasmic reticulum. In ER A subunit of holotoxin is proteolysed to 27KD A1 subunit that binds to 60s ribosomal subunit and cleaves ribosomal RNA. This leads to inhibition of protein synthesis and death of the cell.
- For reasons not known, endothelial cells within renal vasculature are affected. Hence patient suffers from acute renal failure
- Atypical HUS (D- HUS)- is caused due to defect in complement system. Hence Eculizumab is useful in this type of HUS.
- Other causes of HUS include
- Genetic disorders of complement regulation- Factor H, I, MCP, Factor B, C3, thrombomodulin
- Streptococcus pneumoniae
- HIV
- Malignancy
- Defective cobalamin metabolism
- Drugs- Quinine, gemcitabine, bleomycin etc
- Pregnancy, Estrogen use
- Autoimmune diseases such as SLE, APLA etc
- Clinical features:
- Hemorrhagic gastroenteritis followed by oliguria
- CNS manifestations in 25% patients- Confusion, paresis, seizures
- Investigations
- Stool culture
- Stool for Stx toxin
- Serology for E.coli
- Tests for complement proteins
- Treatment
- Supportive care and dialysis
- No great use of plasma exchange/ heparin/ glucocorticoids/ antibiotics
- Renal transplantation if there is end stage renal disease
- Eculizumab for D- cases.
Figures:
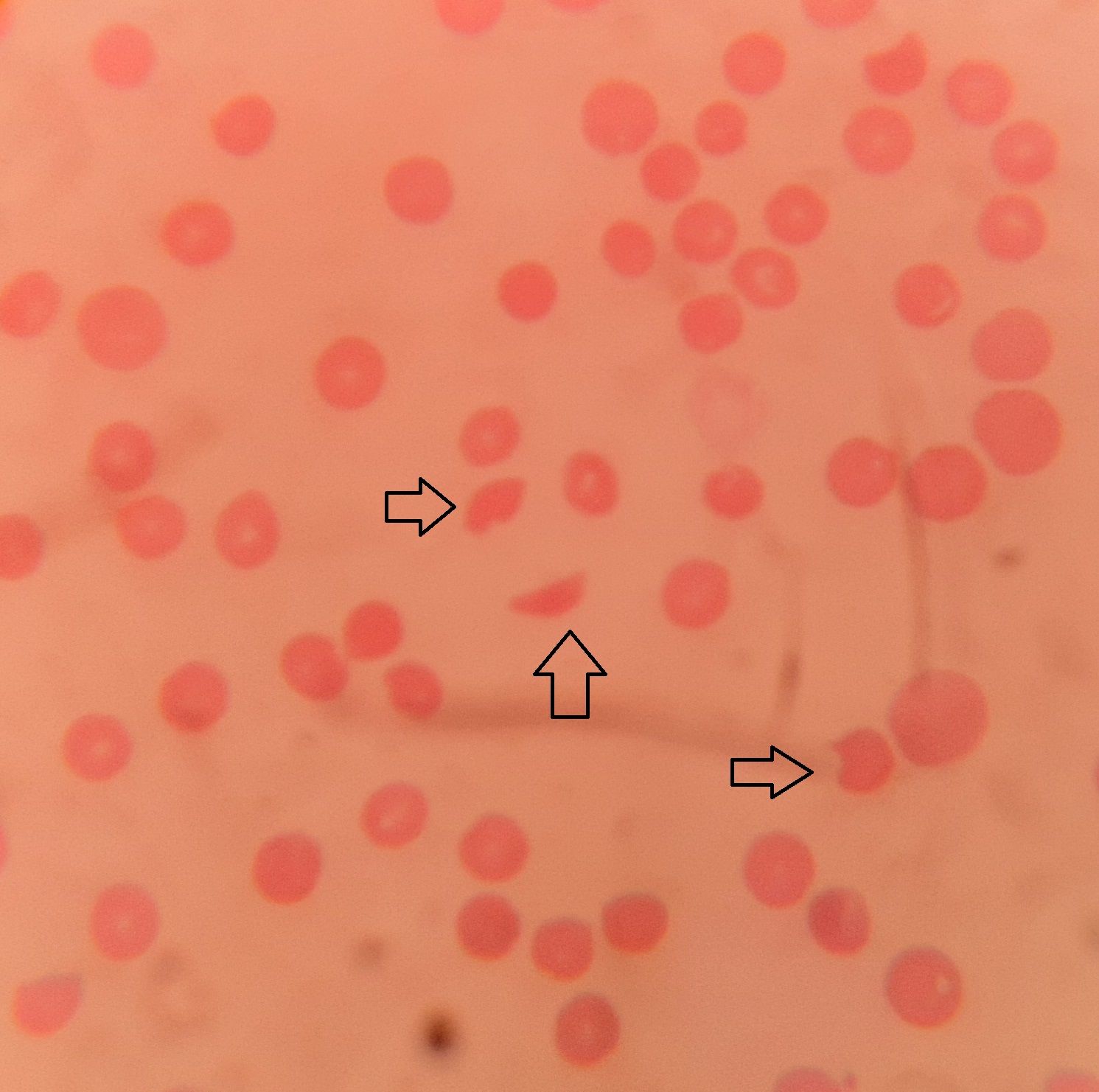
Figure 10.2.1- Schistocytes (arrows) in case of thrombotic thrombocytopenic purpura
Recent advances:
Recombinant ADAMTS13 for Hereditary Thrombotic Thrombocytopenic Purpura
In this case report, a 27-year-old patient with hereditary thrombotic thrombocytopenic purpura had presented with an acute episode in the 30th week of her second pregnancy. When the acute episode of hereditary TTP became plasma-refractory and fetal death was imminent, weekly injections of recombinant ADAMTS13 at a dose of 40 U per kilogram of body weight were initiated. The patient’s platelet count normalized, and the growth of the fetus stabilized. At 37 weeks 1 day of gestation, a small-for-gestational-age boy was delivered by cesarean section.
https://doi.org/10.1056/NEJMoa2211113
Risk of relapse in immune-mediated thrombotic thrombocytopenic purpura and the role of anti-CD20 therapy
Present study reviewed patients with iTTP having had >3 years of follow-up over 10 years in the United Kingdom to identify patient characteristics for relapse, assess relapse rates and patterns, and response to anti-CD20 therapy. Study identified 443 patients demonstrating relapse rates of 40% at 5-year follow-up. The study demonstrated that iTTP diagnosed in the latter part of the study period had lower rates of clinical relapses with the advent of regular monitoring and preemptive rituximab. In ADAMTS13 relapses, 96% responded to anti-CD20 therapy, achieving ADAMTS13 activity of >20%. Anti-CD20 therapy was demonstrated to be an effective long-term treatment regardless of relapse pattern.
https://doi.org/10.1182/blood.2022017023
Obinutuzumab and ofatumumab in thrombotic thrombocytopenic purpura
Obinutuzumab and ofatumumab are humanised anti-CD20 treatments. They were used in patients with severe infusion-related reactions to rituximab, acute rituximab-induced serum sickness and a short duration of disease remission. All patients achieved disease remission (ADAMTS13 activity ≥30 iu/dl) after a median 15 days and 92% of episodes achieved complete remission (≥60 iu/dl). These results suggest that obinutuzumab and ofatumumab may be considered as an alternative option to rituximab in the treatment of TTP.
https://doi.org/10.1111/bjh.18192
Bortezomib, a promising alternative for patients with refractory or relapsed thrombotic thrombocytopenic purpura after rituximab treatment
From October 2017 to October 2021, four refractory/relapsed iTTP patients were enrolled in this study who were refractory to rituximab and cyclosporine. Bortezomib was administered at a dose of 1.3 mg/m2 by subcutaneous injection on days 1, 4, 8 and 11 of a 28-day cycle. All four patients obtained complete remission.
https://doi.org/10.1111/bjh.18430
Bortezomib in relapsed/refractory immune thrombotic thrombocytopenic purpura
In cases of relapsed or refractory TTP, the proteasome inhibitor bortezomib is considered as an immune-modulating therapy, although evidence is primarily based on case reports and series. This study presents the experience with bortezomib in eight patients and conducts a systematic review of literature, identifying 28 additional cases. Following bortezomib treatment, 72% of patients achieved a complete response, and 85% maintained a durable response without relapse at the last follow-up, highlighting its potential efficacy in refractory TTP cases.
https://doi.org/10.1111/bjh.19035
Recombinant ADAMTS13 in Congenital Thrombotic Thrombocytopenic Purpura
In a phase 3 crossover trial, patients with congenital thrombotic thrombocytopenic purpura (TTP) were randomly assigned to receive either recombinant ADAMTS13 or standard therapy for two 6-month periods, followed by the alternate treatment, and then recombinant ADAMTS13 for an additional 6 months. During prophylaxis with recombinant ADAMTS13, no acute TTP events occurred, contrasting with one event during standard therapy. Thrombocytopenia was the most common TTP manifestation, with lower rates observed with recombinant ADAMTS13. Adverse events were less frequent and less severe with recombinant ADAMTS13 compared to standard therapy, with no drug-related adverse events leading to trial-drug interruption or discontinuation. The study concludes that recombinant ADAMTS13 prophylaxis effectively raised ADAMTS13 activity levels to near-normal levels, resulting in rare TTP events and manageable adverse events.
https://doi.org/10.1056/NEJMoa2314793
Management of immune thrombotic thrombocytopenic purpura without therapeutic plasma exchange
This retrospective study compared outcomes of acute immune thrombotic thrombocytopenic purpura (iTTP) patients treated with caplacizumab and immunosuppression, either with or without therapeutic plasma exchange (TPE). The results showed no significant difference in time to platelet count normalization, clinical response, or iTTP-related deaths between the two groups, suggesting that caplacizumab, without TPE, can effectively control thrombotic microangiopathy. The study supports TPE-free management for iTTP in experienced centers, highlighting the importance of individualized decision-making between physicians and patients.
https://doi.org/10.1182/blood.2023023780
Daratumumab in multiresistant immune-mediated thrombotic thrombocytopenic purpura
This nationwide survey evaluated daratumumab for immune-mediated thrombotic thrombocytopenic purpura (iTTP) in patients intolerant or refractory to rituximab. Among nine episodes in seven patients, daratumumab improved ADAMTS13 activity in eight cases, with normalization in three. Retreatment with daratumumab was successful in two of three relapsed patients, with a 12-month ADAMTS13 relapse-free survival of 56%. These findings suggest daratumumab as a promising option for iTTP management, with manageable infusion-related side effects.
https://doi.org/10.1111/bjh.19752
An Initiative of
Veenadhare Edutech Private Limited
1299, 2nd Floor, Shanta Nivas,
Beside Hotel Swan Inn, Off J.M.Road, Shivajinagar
Pune - 411005
Maharashtra – India
howitreat.in
CIN: U85190PN2022PTC210569
Email: admin@howitreat.in
Disclaimer: Information provided on this website is only for medical education purposes and not intended as medical advice. Although authors have made every effort to provide up-to-date information, the recommendations should not be considered standard of care. Responsibility for patient care resides with the doctors on the basis of their professional license, experience, and knowledge of the individual patient. For full prescribing information, including indications, contraindications, warnings, precautions, and adverse effects, please refer to the approved product label. Neither the authors nor publisher shall be liable or responsible for any loss or adverse effects allegedly arising from any information or suggestion on this website. This website is written for use of healthcare professionals only; hence person other than healthcare workers is advised to refrain from reading the content of this website.